Published online Apr 28, 2022. doi: 10.3748/wjg.v28.i16.1608
Peer-review started: August 25, 2021
First decision: September 29, 2021
Revised: October 6, 2021
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: April 28, 2022
Supralevator, suprasphincteric, extrasphincteric, and high intrarectal fistulas (high fistulas in muscle layers of the rectal wall) are well-known high anal fistulas which are considered the most complex and extremely challenging fistulas to manage. Magnetic resonance imaging has brought more clarity to the patho
Core Tip: These are the first published guidelines to manage the five types of peri-levator anal fistulas that involve almost the complete external anal sphincter and have, therefore, been grouped together as high-5 fistulas. These are supralevator, suprasphincteric, extrasphincteric, and high intrarectal and fistulas at the roof of ischiorectal fossa inside the levator ani muscle. The diagnosis and management of these five fistulas is quite challenging. Magnetic resonance imaging is the best modality to accurately delineate these fistulas. Once diagnosed, care should be exercised to avoid sphincter-cutting procedures (fistulotomy) in these fistulas, as the risk of incontinence would be very high. Sphincter-sparing procedures should be done. However, there is little literature available on satisfactory management of these fistulas.
- Citation: Garg P, Yagnik VD, Dawka S, Kaur B, Menon GR. Guidelines to diagnose and treat peri-levator high-5 anal fistulas: Supralevator, suprasphincteric, extrasphincteric, high outersphincteric, and high intrarectal fistulas. World J Gastroenterol 2022; 28(16): 1608-1624
- URL: https://www.wjgnet.com/1007-9327/full/v28/i16/1608.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i16.1608
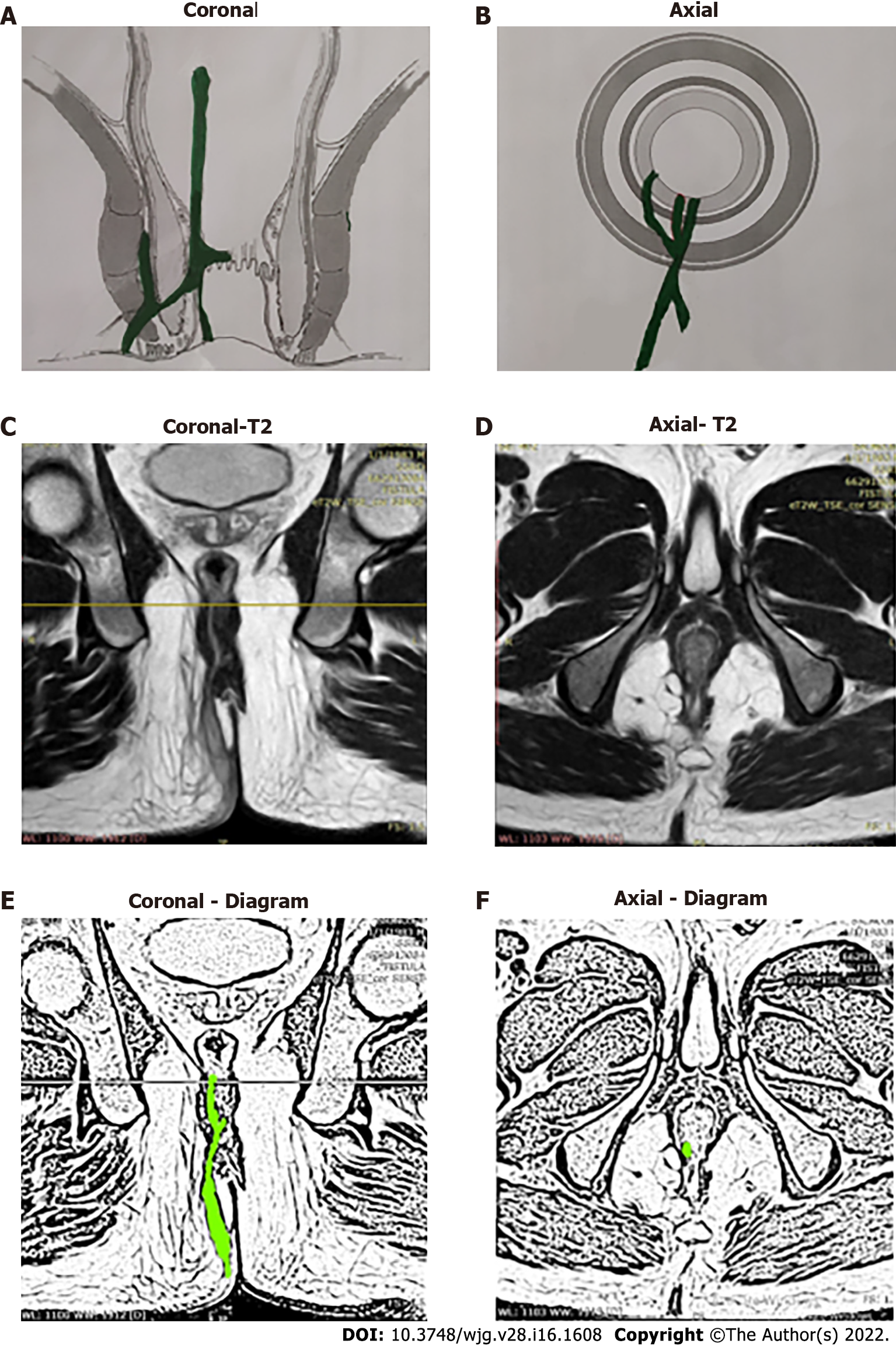
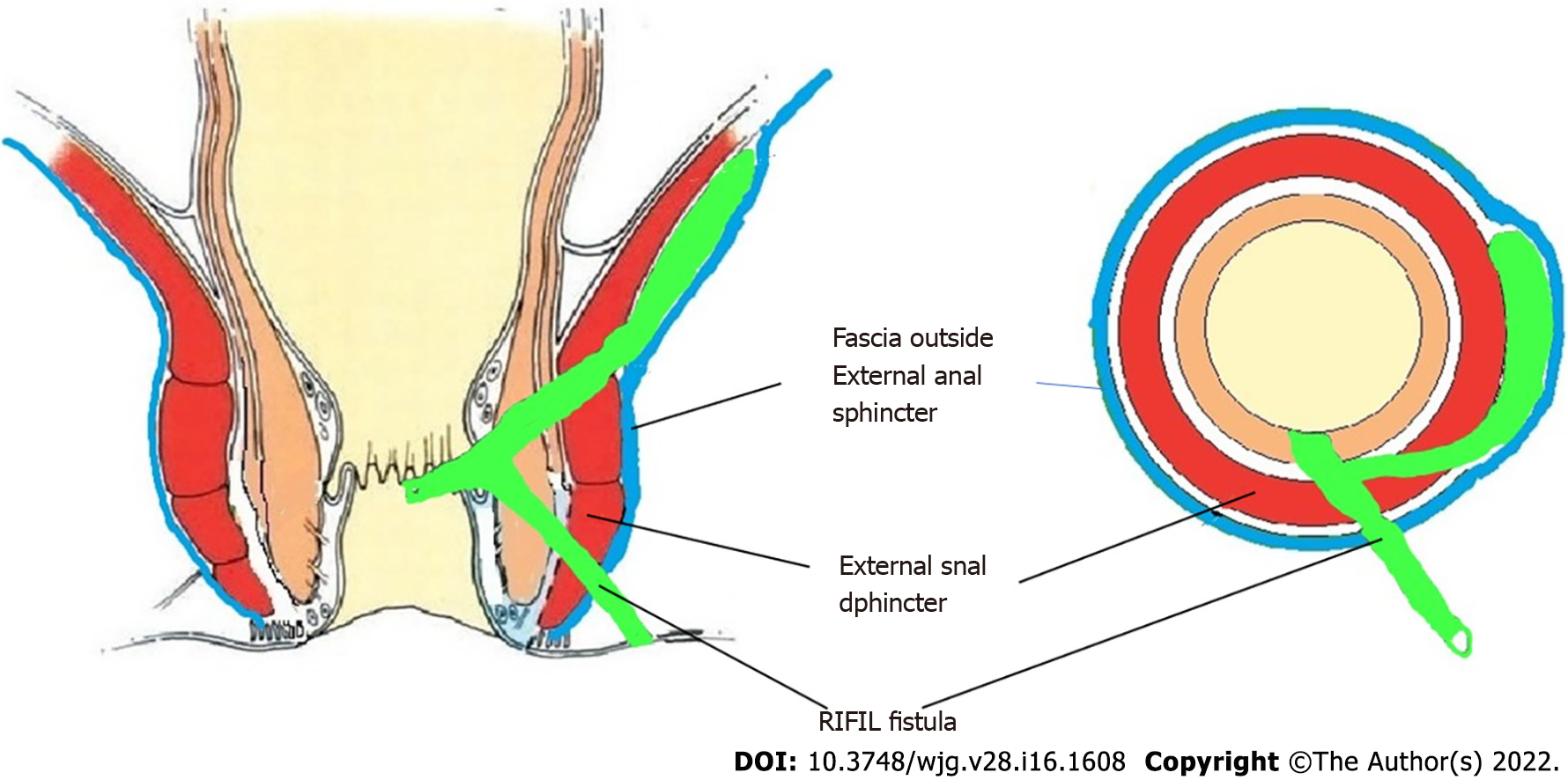
Anal fistulas are feared by patients and surgeons alike[1]. The prime reasons for this are difficulty in understanding the pathophysiology, risk of debilitating incontinence, and high recurrence rates, especially in complex fistulas[2-5]. Amongst the category of complex fistulas, the three well-known notorious fistulas are supralevator, suprasphincteric and extrasphincteric fistulas[6,7]. Another less known fistula is the high intra-rectal fistula which occurs by cephalad extension of the fistula between the internal anal sphincter (IAS) and conjoint longitudinal muscle (CLM). This fistula was first described by Parks et al[8] in 1976. Recently, another fistula, fistula at the roof of ischiorectal fossa inside the levator ani muscle (RIFIL) has also joined the category of these high fistulas[9]. RIFIL fistulas are also highly complex and involve the complete external anal sphincter (EAS). The common feature of these five fistulas is that they are quite high as these reach up to the levator muscle and involve almost the complete EAS (Figure 1). Hence, these five fistulas have been grouped together as high-5 fistulas and are discussed in detail below.
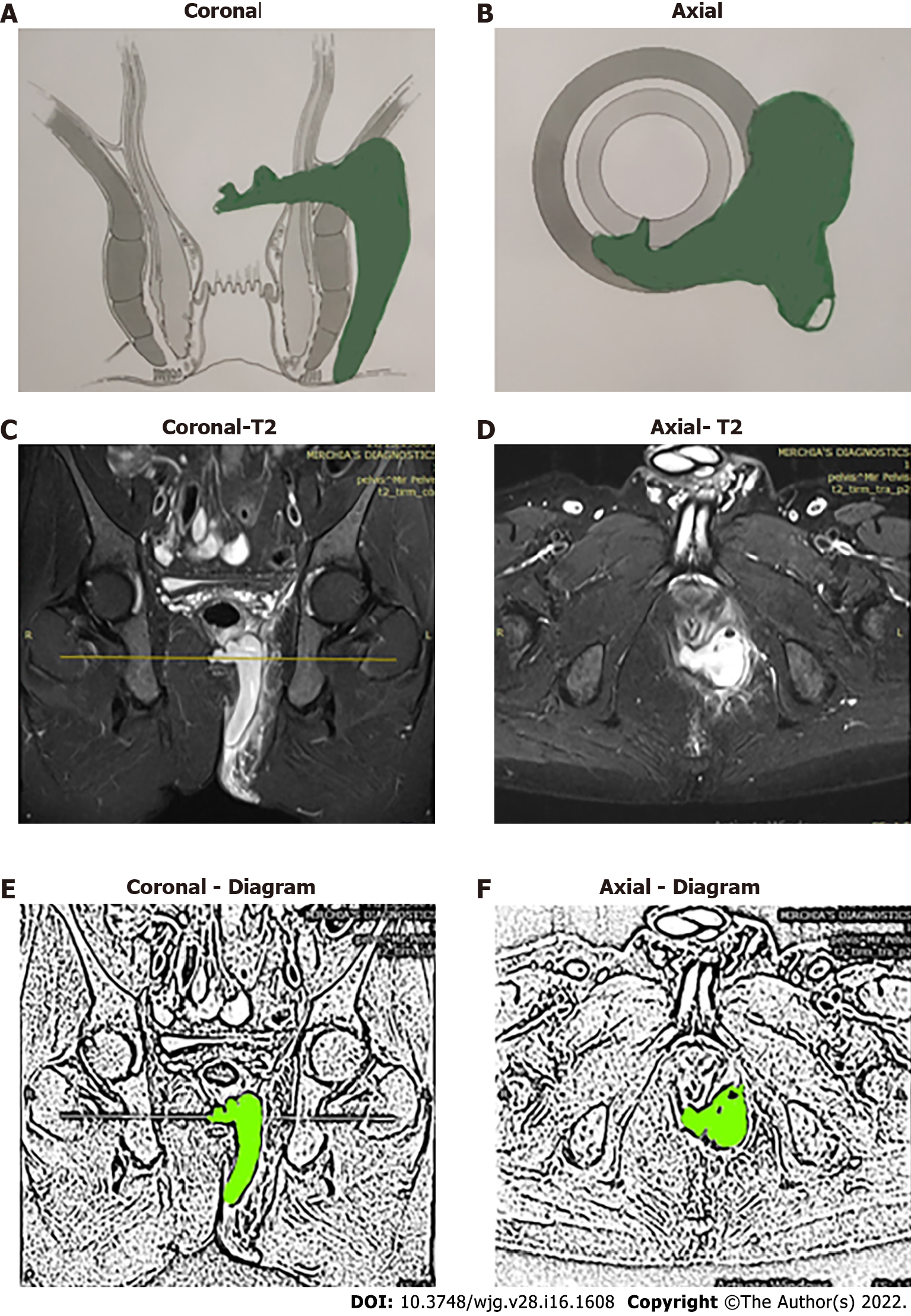
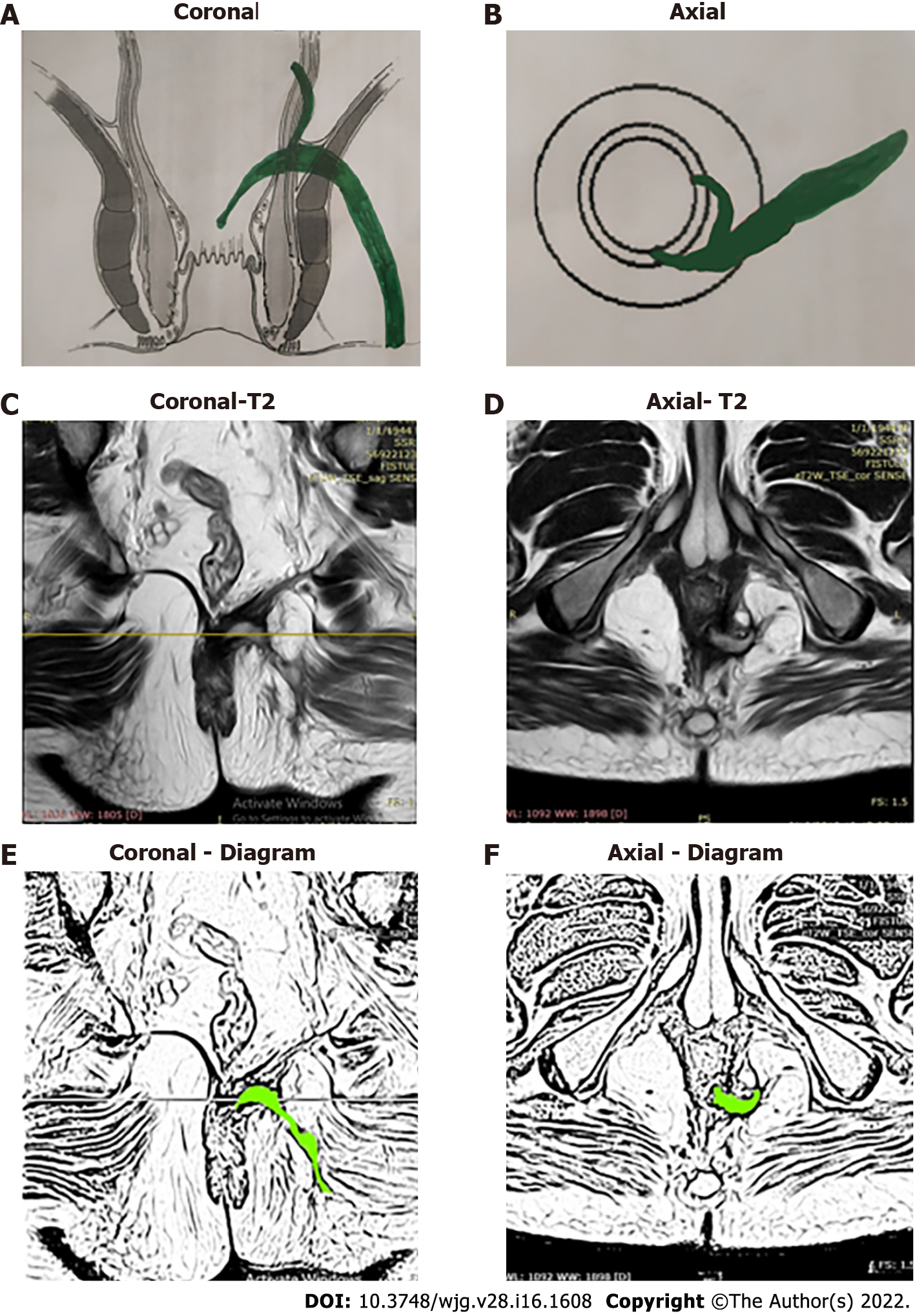
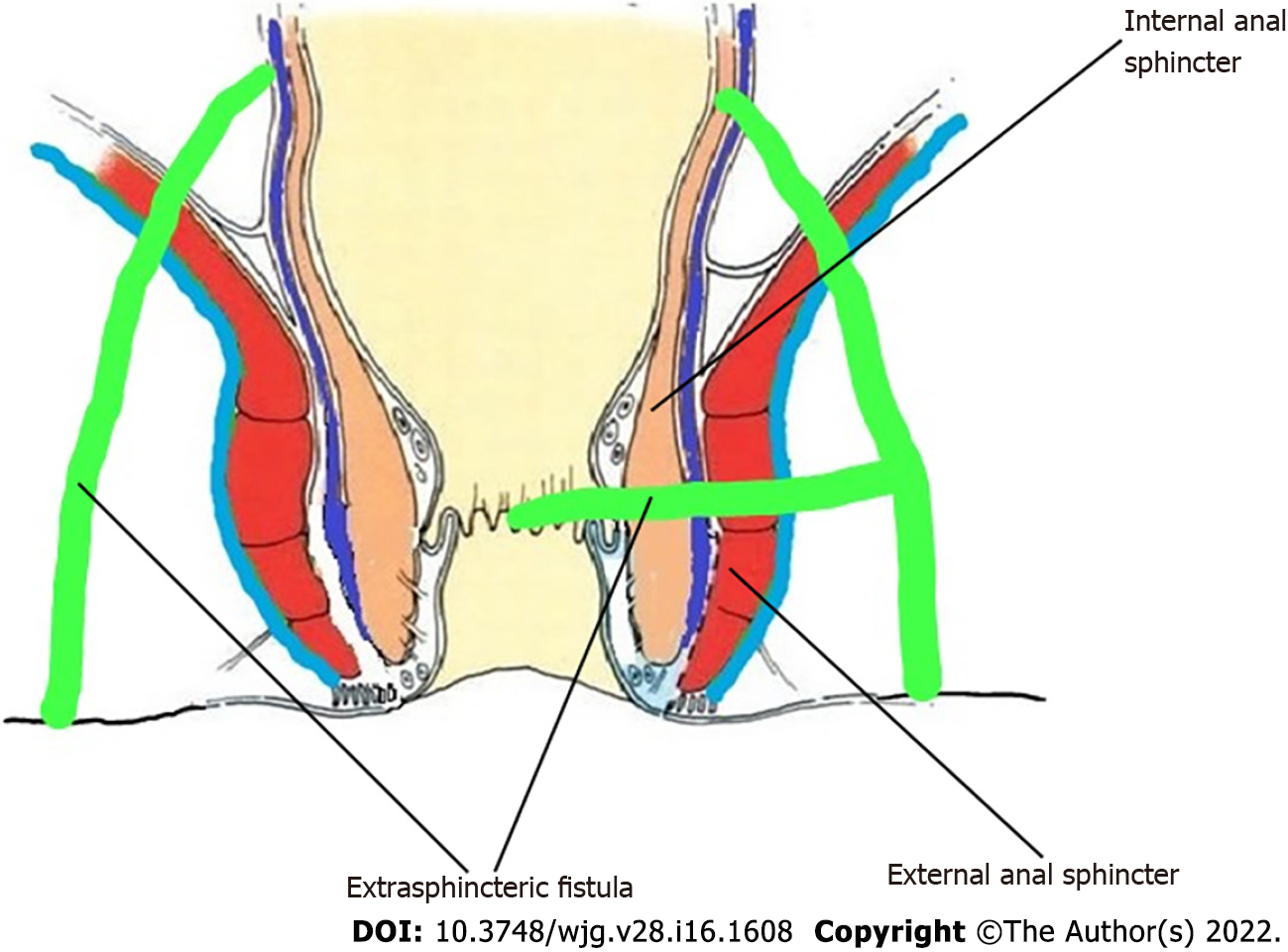
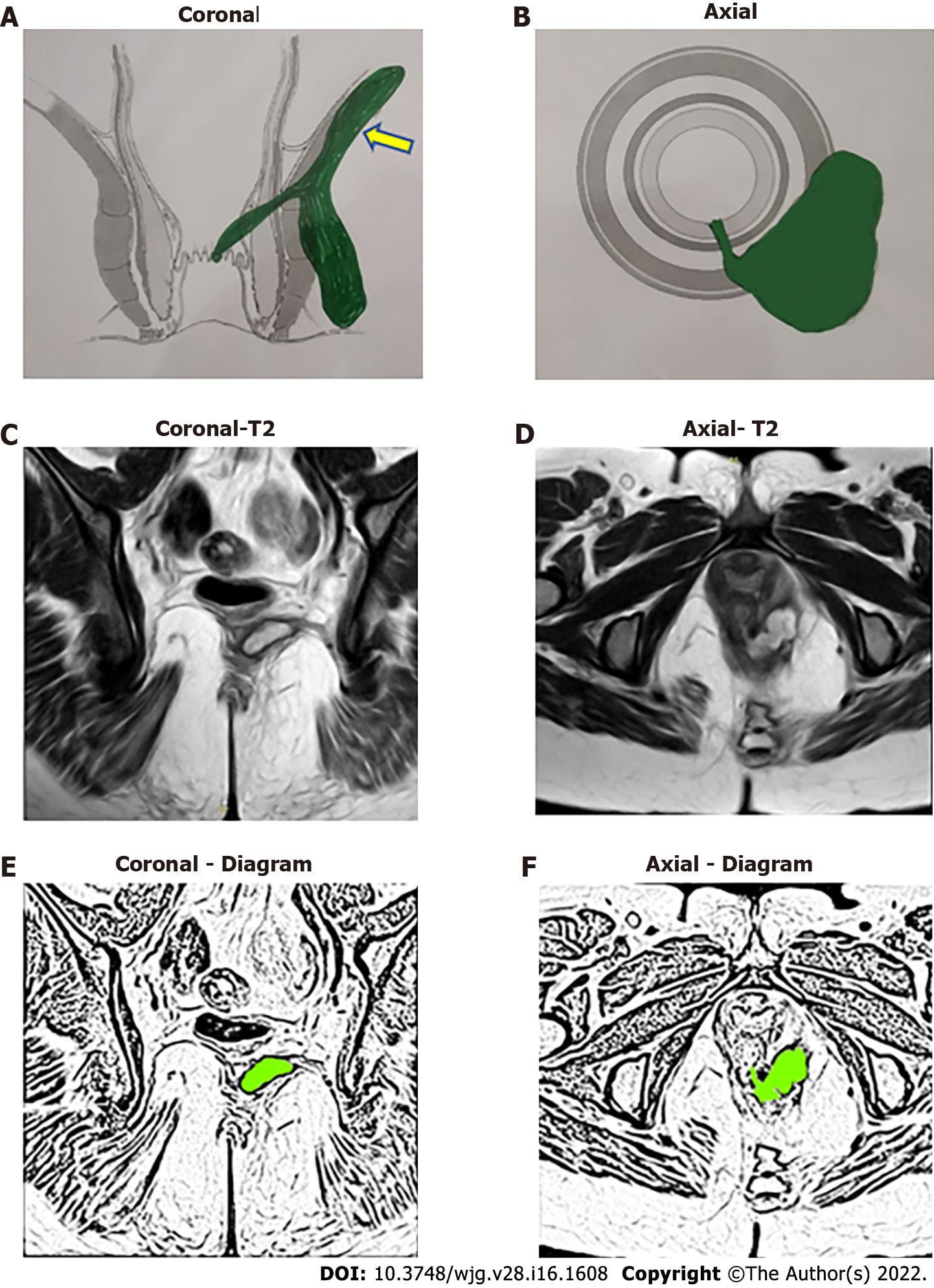
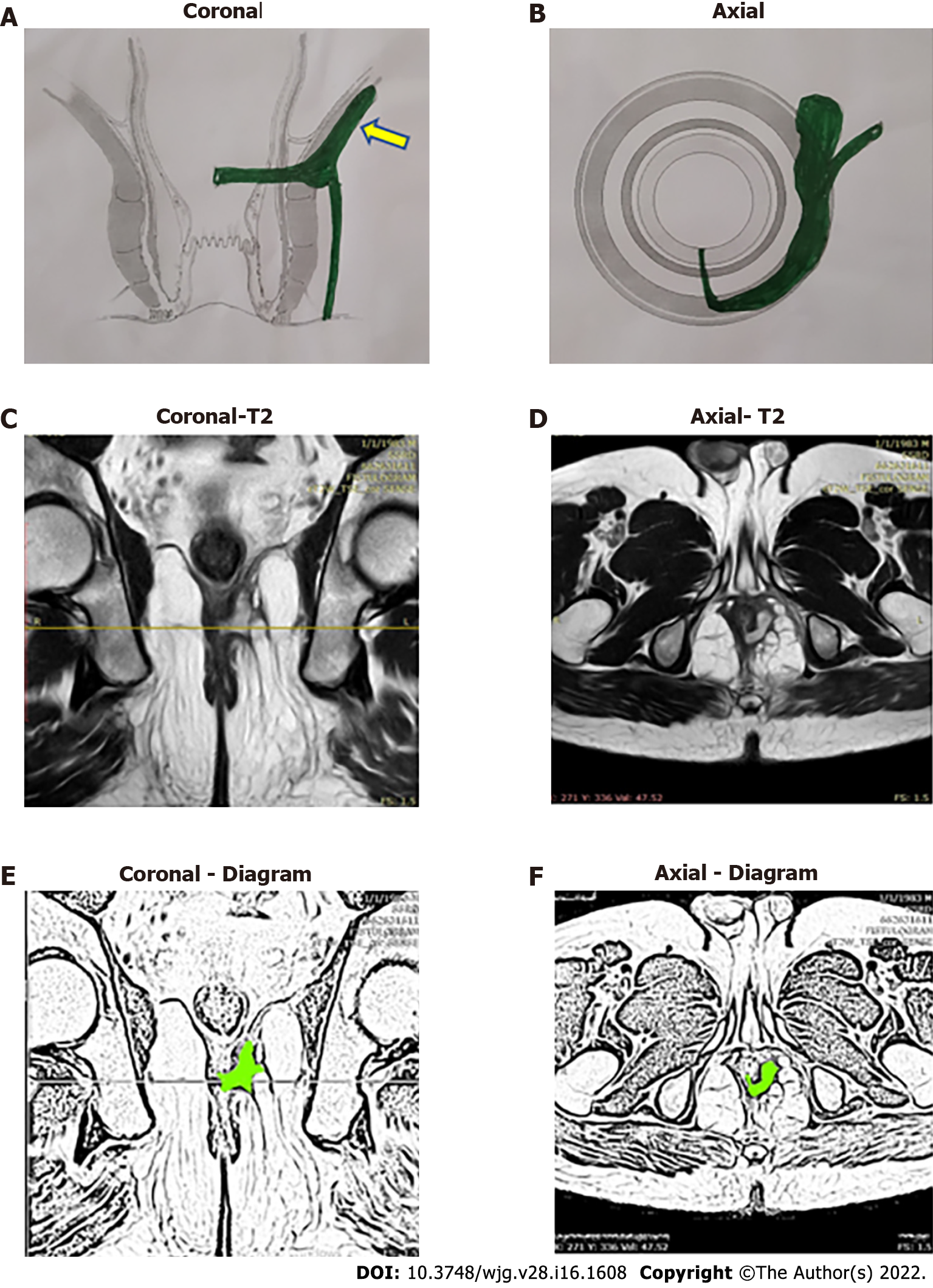
The high-five anal fistulas include: (1) Supralevator (Figures 2 and 3); (2) Suprasphincteric (Figures 4-6); (3) Extrasphincteric (Figure 7); (4) High intra-rectal (Figures 3,8 and 9)[8]; and (5) RIFIL (Figures 10-12)[9]. These fistulas are considered most dreaded because understanding their anatomy and pathophysiology, proper diagnosis, and successful safe treatment are quite challenging. All these five fistulas involve almost the complete EAS[10,11] (Figures 2-12). As the EAS plays a major role in continence, muscle-dividing procedures like fistulotomy or cutting setons are strictly contraindicated in these fistulas. In fact, fistulotomy with primary sphincter reconstruction or fistulectomy with primary sphincter reconstruction are also best avoided in these patients[1,12-14]. Therefore, the choice of procedures becomes limited. Of these, the extrasphincteric is the only type which is trans-levator, whereas the other four fistulas (supralevator, sphincteric, high intrarectal, and RIFIL) are peri-levator fistulas which do not traverse through the levator muscle.
This article will address the major concerns of these fistulas, including the challenges in managing and diagnosing the high-5 fistulas, as well as methods to formulate management guidelines..
There is a lot of confusion amongst surgeons about the exact anatomy, pathways of spread, and extent of these fistulas[2]. Not uncommonly, a high transsphincteric fistula is labelled as an extrasphincteric fistula and a suprasphincteric fistula is reported as a high transsphincteric fistula[2].
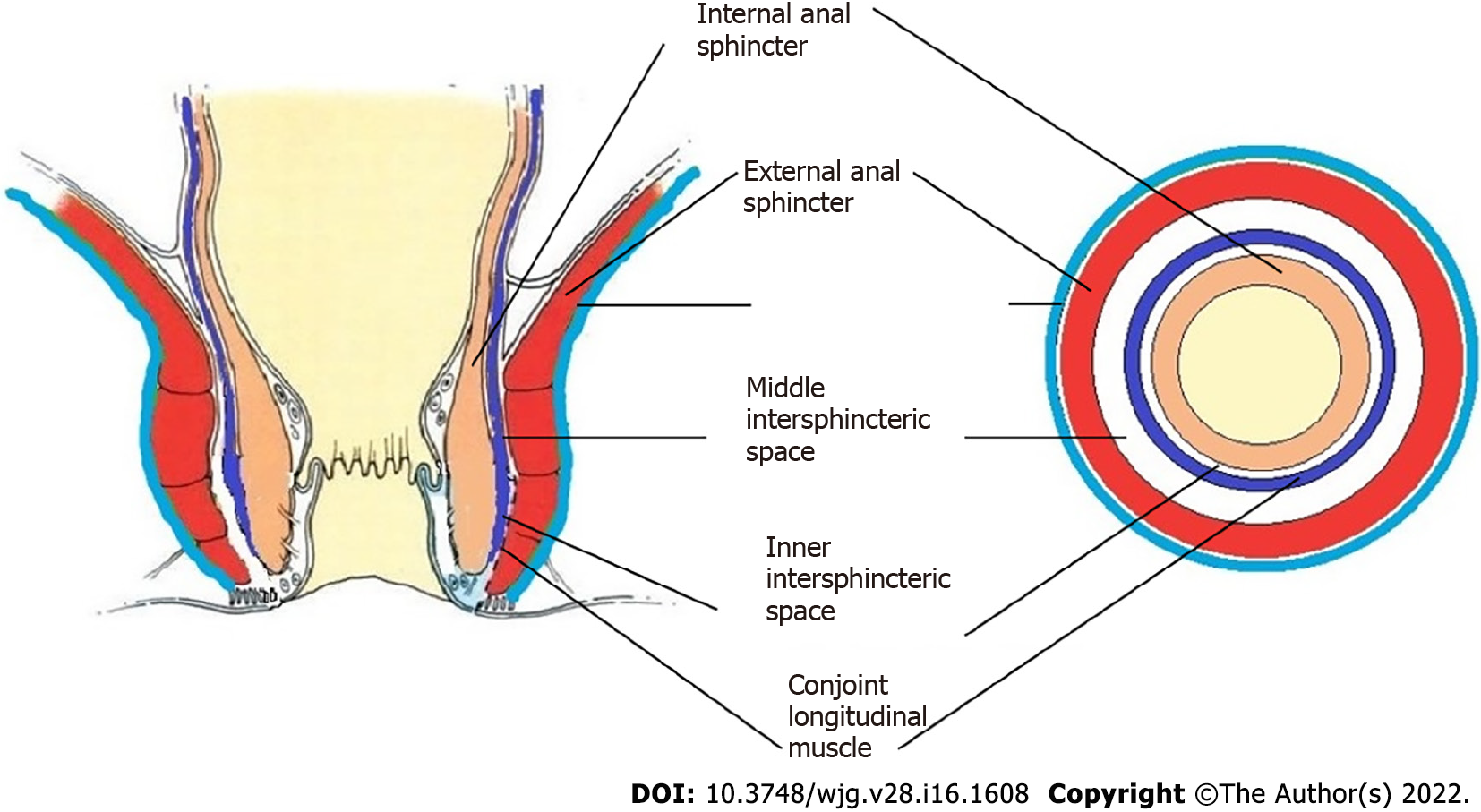
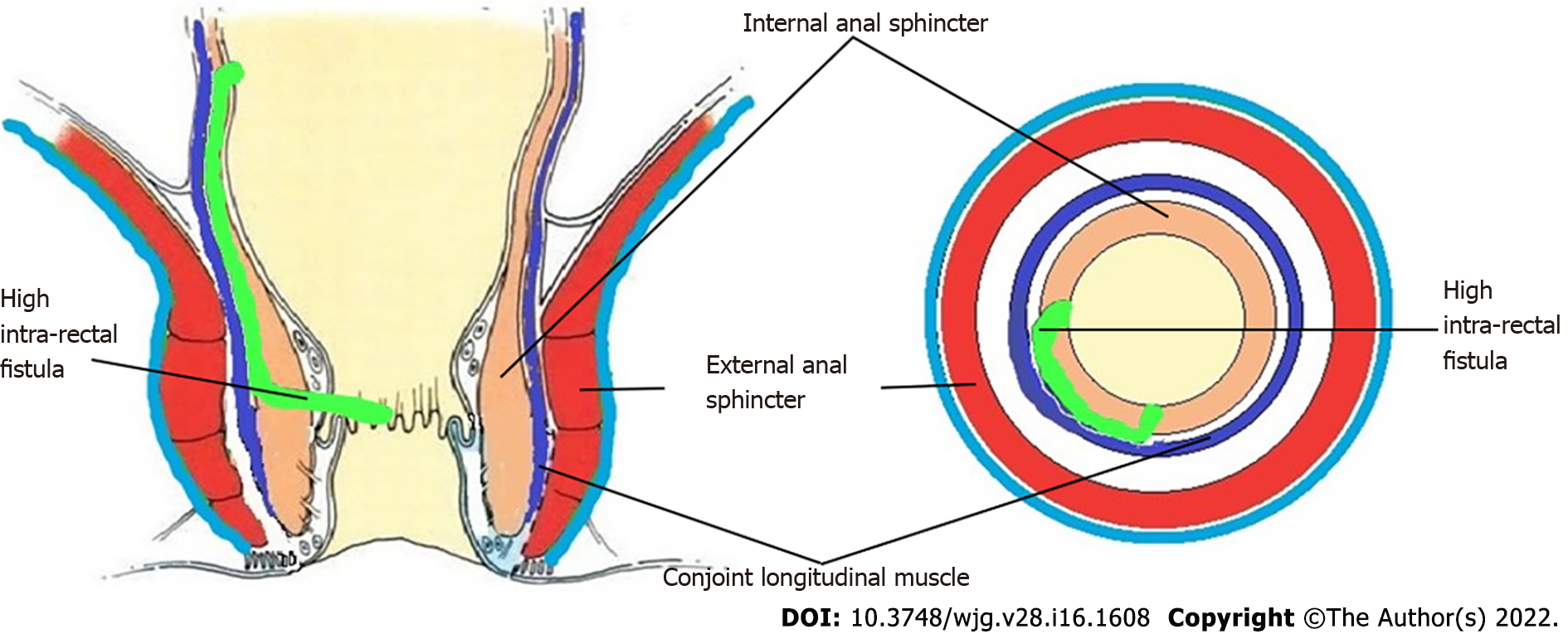
In order to understand the pathways of extension of these fistulas, it is important to understand the anatomy of the anal canal. There are three muscle layers in the sphincter-complex in the anorectum which are downward extensions of the muscle layers of the gut. There are potential spaces between these muscle layers in which the fistula and pus can spread[9] (Figures 1, 2, 4, 7, 8 and 10).
The three muscle layers in the sphincter-complex[9] are (1) Inner circular muscle layer of gut continues downwards in the anal sphincter complex as IAS; (2) Outer longitudinal muscle layer of gut continues downwards in the anal sphincter complex as CLM; and (3) Puborectalis component of levator ani muscle continues downwards in the anal sphincter complex as EAS (Figure 1). Conventionally, it was assumed that inside the sphincter-complex, there is only one space where the fistula extends and this space between the IAS and EAS was labelled as the ‘intersphincteric space’. However, with the availability of higher resolution magnetic resonance imaging (MRI) and increasing experience, it has been demonstrated that there are three potential spaces associated with in these muscle layers through which the fistula can extend[9].
The three potential spaces in the anal sphincter-complex are (1) Inner intersphincteric space (inner space): Between IAS and CLM; (2) Middle intersphincteric space (middle space): Between CLM and EAS (conventional intersphincteric space); and (3) Outer-sphincteric space (outer space): Between the lateral muscle fibers of EAS and its covering fascia[9] (Figure 1).
The anal fistula, usually initiating at crypt glands at the level of the dentate line, can extend superiorly (cephalad) in any of these three spaces.
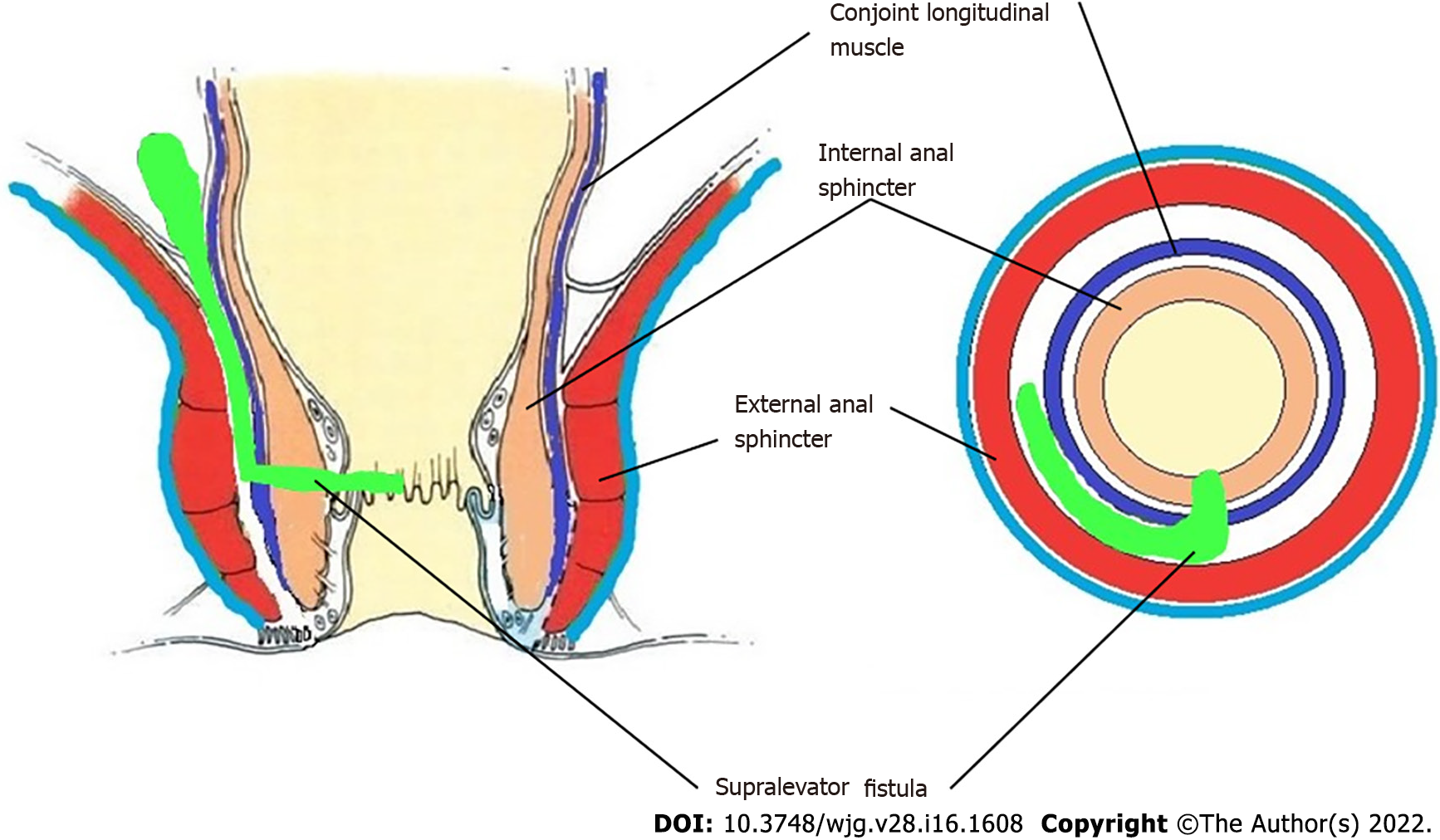
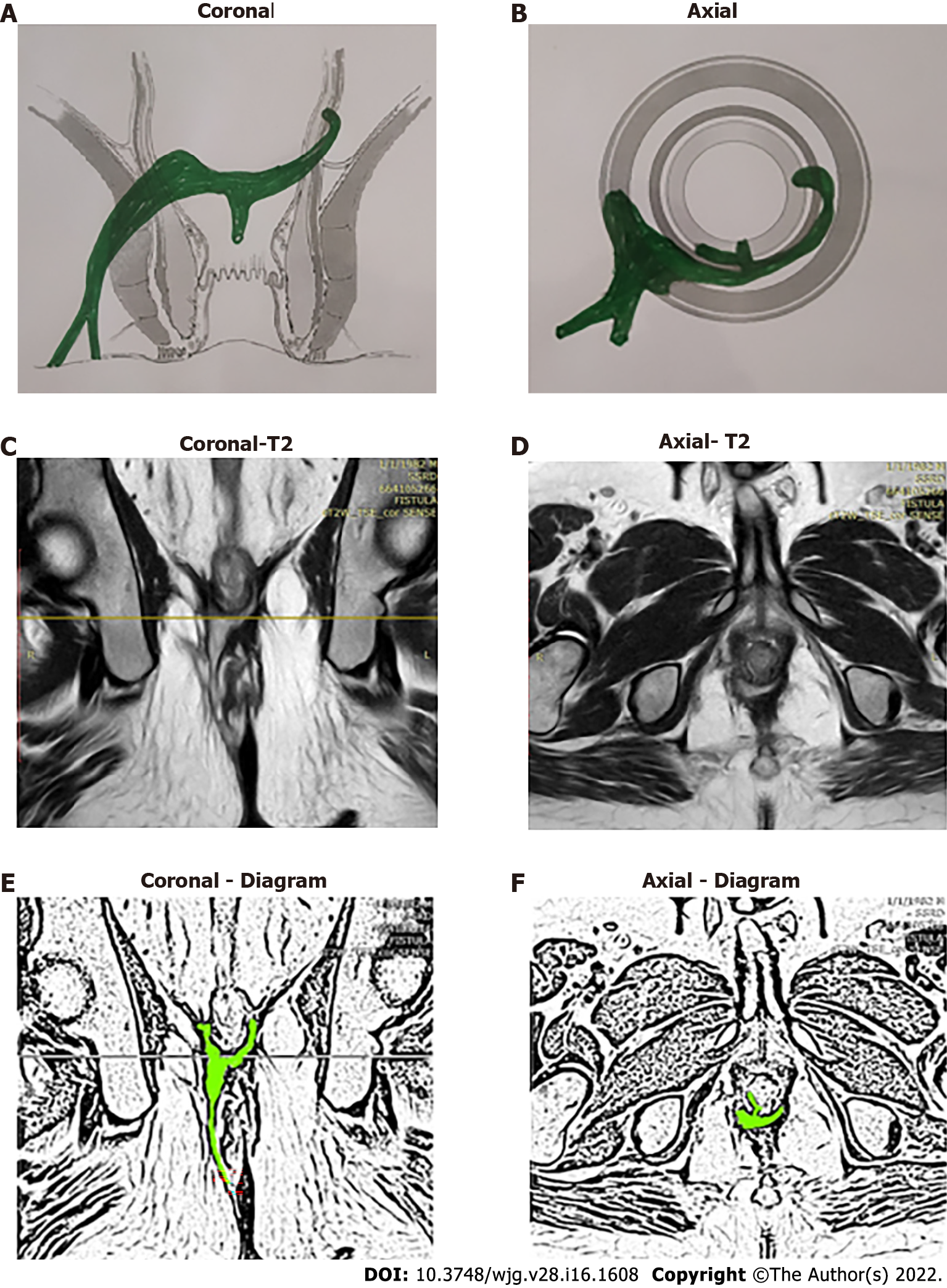
The fistulas traversing superiorly in (1) Inner intersphincteric space (inner space) between IAS and CLM inside the rectal wall to become a high intrarectal fistula as these fistulas are present between the muscle layers of the rectal wall[8]. At times, these are erroneously labelled as submucous fistula[8] (Figures 8 and 9); (2) Middle intersphincteric space (middle space) between CLM and EAS become a supralevator fistula (if it continues into the supralevator space) (Figures 2 and 3) or a suprasphincteric fistula (if pus extending superiorly in the middle space reaches up to the top of the EAS and then traverses through the junction between the EAS and puborectalis to enter the ischiorectal fossa[15]) (Figures 4-6); and (3) Outer-sphincteric space (outer space) between the EAS and its lateral fascia becomes a RIFIL fistula[9] (Figures 10-12).
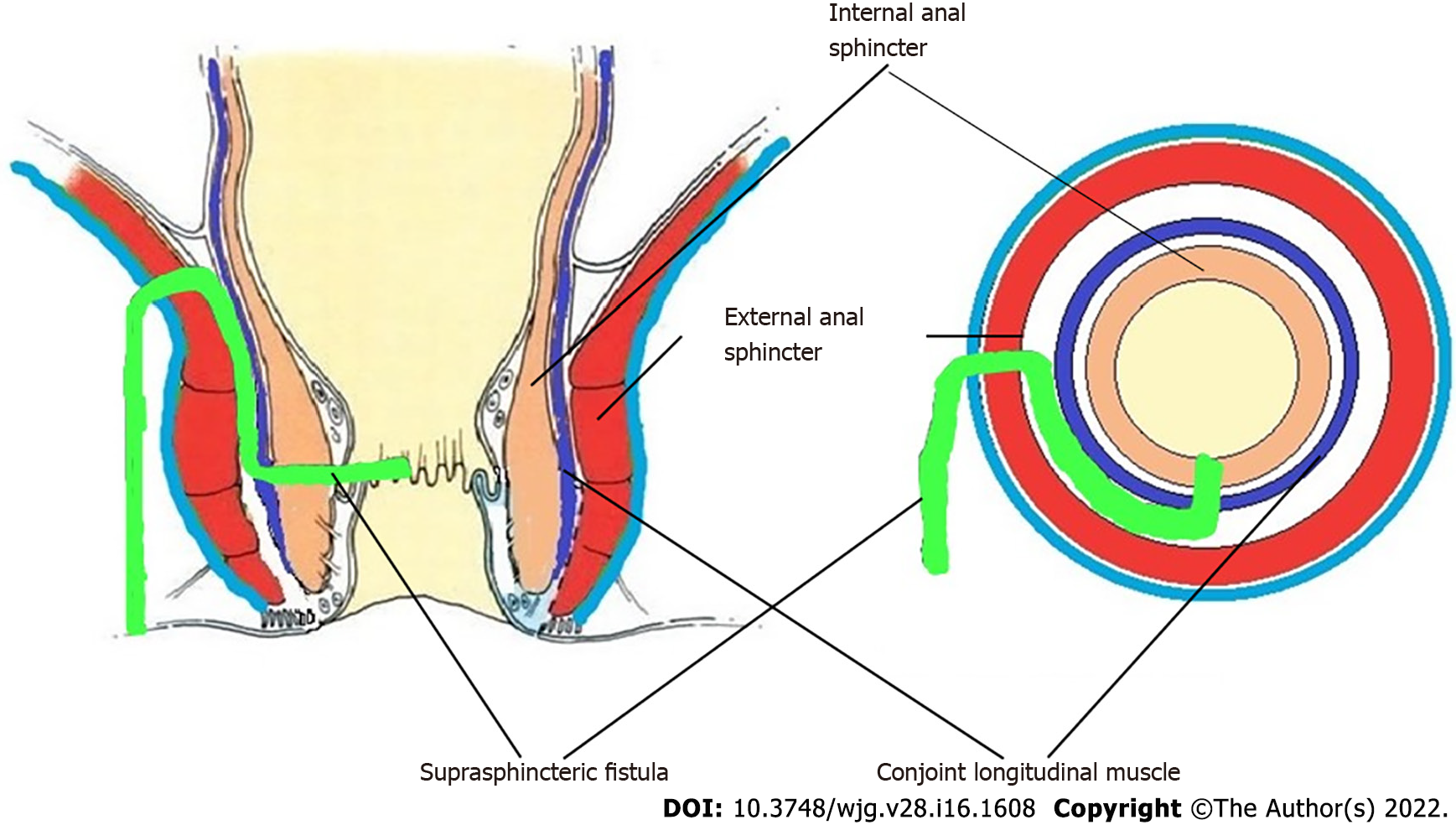
Thus, the definition of these five fistulas is: (1) Supralevator (Figures 2 and 3): These fistulas traverse in the middle intersphincteric space to enter the supralevator space. The fistula may open into the rectum through an additional high rectal opening in the supralevator space (Figures 3 and 6); (2) Suprasphincteric (Figures 4-6): These fistulas extend superiorly in the middle intersphincteric space to reach up to the top of the EAS and then traverses through the junction between the EAS and puborectalis to enter the ischiorectal fossa; (3) Extrasphincteric (Figure 7): These fistulas traverse through the ischiorectal fossa and enter the supralevator space penetrating the levator muscle. They generally do not traverse through the sphincter-complex (EAS and IAS), which is why they are labelled as ‘extra-sphincteric’ fistulas; (4) High intra-rectal (Figures 8 and 9): These fistulas traverse cephalad between IAS and CLM in the rectal wall and is thus present between the muscle layers of the rectal wall; and (5) RIFIL (Figures 10-12): These fistulas traverse the EAS but do not enter the ischiorectal fossa. They extend cephalad between the EAS and its lateral fascia to continue on the undersurface of puborectalis and levator ani muscle[9].
Incidence: In recently published large cohorts, the prevalence of these high-5 fistulas has been highlighted[9,16]. The incidence of these fistulas in a cohort of 419 consecutively operated patients over a 2-year period were RIFIL–10% (42/419), supralevator-9.5% (40/419), suprasphincteric-5.5% (23/419) and extrasphincteric-0%[9,16].
As these fistulas are deep and high, they are extremely difficult to diagnose on clinical examination. It usually requires advanced imaging modalities, MRI, or transrectal ultrasound (TRUS), to diagnose these fistulas[17-21]. Both MRI and 3D-TRUS are very effective in delineating various parameters of these fistulas, including the internal opening, primary tracts, and secondary extensions[17-20,22], though MRI has a slight edge over TRUS. It is really impressive that in the pre-MRI era, doyens in the field like Parks et al[8] could describe these deep fistulas with a reasonably high level of accuracy.
MRI helps to clearly differentiate between supralevator, suprasphincteric, high intrarectal, and RIFIL fistulas[23]. Rather it would not be wrong to say that MRI is mandatory to properly diagnose these fistulas. Therefore, whenever a fistula has recurred several times, or a tract or side branch of the main fistula is seen extending superiorly during surgery, an MRI should be done to rule out these fistulas[23].
Increasing experience with MRI in fistulas has highlighted that extrasphincteric fistulas do not occur or are extremely rare[24]. It stands to reason that it is almost impossible for a fistula in the ischiorectal fossa to penetrate through the strong levator plate when it can extend with ease in the surrounding fat of the ischiorectal fossa[24]. The only likely possibility for an extrasphincteric fistula to occur is iatrogenic when an artery forceps in a high transsphincteric fistula is pushed through the levator muscle. Even this seems improbable as it would require a lot of force by the operating surgeon. Moreover, due to increased awareness amongst surgeons of iatrogenic injuries, better understanding of regional anatomy, and easy availability of advanced radiological modalities, these iatrogenic blunders seem exceedingly unlikely. Due to this, extrasphincteric fistulas are not seen (or are extremely rare) these days[24]. It is quite possible that the fistulas diagnosed as extrasphincteric fistulas in the pre-MRI era were perhaps supralevator or RIFIL fistulas. Understandably, in the absence of MRI, it is extremely difficult to differentiate between an extrasphincteric fistula and other deep fistulas. So, a diagnosis of extrasphincteric fistula should be made only after due deliberation and detailed analysis of MRI[24].
The main challenge in these five fistulas is that they involve almost the complete EAS (Figures 2, 4, 7, 8 and 10). The EAS plays a major role in maintaining continence and risk of damage to the EAS is very high in these fistulas. Because this risk of incontinence is so high, these fistulas are extra concerning[4,5,25]. The most commonly performed procedure, fistulotomy, is contraindicated in these fistulas[25]. There is little published data and there are no standard guidelines to manage these fistulas. Therefore, the fear of managing these fistulas amongst surgeons in easy to understand.
One of the features that make these fistulas more challenging is the presence of an ASRO along with a primary internal opening at the dentate line[6]. In a large cohort, ASRO was present in 2.8% (23/836) anal fistulas, but in supralevator fistulas, the incidence was 16.6% (23/138)[6]. At times, the presence of ASRO is detected accidentally during surgery (intraoperatively) when a colored solution injected into the external opening comes out through the primary internal opening at the dentate line as well as through the ASRO[6]. In many cases, a granulation polyp or a papilla can be seen at the site of the ASRO. However, ASRO can be diagnosed preoperatively with the help of MRI in almost every case[6]. MRI is an excellent tool to diagnose ASRO with a high level of accuracy[6].
The management of ASRO seems challenging. Closure of an opening high up in the rectum which, at times, is even difficult to reach through the transanal route is not easy. However, there is a sliver of relief in these otherwise complex fistulas. A recent study has demonstrated that even if nothing is done to ASRO, there is no impact on ultimate fistula healing[6].
There is an explanation for this surprising finding. Anal fistulas usually initiate in crypt glands located at the dentate line. The fistula then can extend in different directions from here. Some fistulas or abscesses extend superiorly and enter the supralevator space[6]. In a small subset (16%) of these supralevator abscesses, the pressure generated by the supralevator abscess is so high that the abscess ruptures into the rectum, thus creating an ASRO. In such situations, the flow of infection (and bacteria) in ASRO is from the supralevator space into the rectum[6]. This continues in the same manner because, unlike in the anal canal where intraluminal pressure rises during defecation, pressure in the mid-rectum is quite low (as the rectum is primarily a storage organ). Therefore, the bacteria usually enter the fistula tract from the primary internal opening at the dentate line, bacterial multiplication leads to suppuration, and then the pus egresses through the external opening and the ASRO (if present)[6]. Hence, ultimate healing of the fistula depends primarily on successful closure of the primary internal opening at the dentate line. Once the primary internal opening at the dentate line heals, the fistula heals irrespective of whether the ASRO is closed or open[6]. Therefore, it is a reassuring fact that ASRO left untreated does not affect the ultimate fistula outcome[6].
The high-5 fistulas are more complex and refractory to treatment as compared to other fistulas. The incidence of associated complicating pathology like Crohn’s disease and tuberculosis is higher in these fistulas[24,26,27]. Therefore, it is important that these diseases should be ruled out whenever any of these high fistulas are diagnosed or suspected.
In regions where Crohn’s disease is common, a colonoscopy should be done in all cases of high fistulas[28]. On the other hand, in regions where tuberculosis is common, polymerase chain-reaction (PCR) should be done in samples from the fistula (pus or fistula tract wall or tract lining)[24,29]. In a large series published from a TB-endemic region (India), tuberculosis was detected in 10% (133/1336) of tested samples (fistula tract lining or pus)[29]. The detection rate of tuberculosis was 12.5% (129/1034) by PCR, 1.5% (3/197) by histopathology, and 0.9% (1/105) by GeneXpert tests[29]. Therefore, PCR is significantly more sensitive than histopathology or GeneXpert to detect tuberculosis[29]. However, in spite of high sensitivity, it is not uncommon that tuberculosis is missed and goes undetected in the first sample. Therefore, in regions where tuberculosis is endemic, it is recommended that repeated samples should be sent for testing, especially in more complex refractory fistulas[29].
A search was performed on MEDLINE, PubMed, EMBASE, and the Cochrane Database of Collected Reviews from January 1975 to September 2021. Keyword combinations using MeSH terms included supralevator, suprasphincteric, extrasphincteric, intrarectal, abscess, fistula, fistula-in-ano, anal, rectal, perianal, perineal, seton, fistula plug, fibrin glue, advancement flap, tuberculosis, Crohn’s disease, ligation of intersphincteric tract, ligation of intersphincteric fistula tract (LIFT), fistulectomy with primary sphincter reconstruction (FPR), fistulectomy with primary sphincter repair, transanal opening of the intersphincteric space (TROPIS) and stem cells.
Various guidelines, such as GRADE[30,31], RIGHT[32] AGREE[33] were evaluated, but considering the rarity of the disease condition (Hi-5 fistulas) in the study, the Levels of Evidence for Therapeutic Studies developed by Centre for Evidence-Based Medicine, http://www.cebm.net. (Oxford, United Kingdom) (Table 1) and Grade Practice Recommendations recommended by American Society of Plastic Surgeons (Table 2) were utilized[34]. Each diagnostic and therapeutic intervention was assigned a ‘level of evidence’ from 1A to 5 (1A being the strongest evidence and 5 being the weakest) (Table 1) and then a ‘grade of recommendation’ was awarded ranging from ‘A’ to ‘D’ (‘A’ being a strong recommendation and ‘D’ being a weak option) (Table 2).
| Level | Type of evidence | |
| 1 | A | Systematic review (with homogeneity) of RCTs |
| B | Individual RCT (with narrow confidence intervals) | |
| C | All or none study | |
| 2 | A | Systematic review (with homogeneity) of cohort studies |
| B | Individual Cohort study (including low quality RCT, e.g., < 80% follow-up) | |
| C | “Outcomes” research; Ecological studies | |
| 3 | A | Systematic review (with homogeneity) of case-control studies |
| B | Individual Case-control study | |
| 4 | Case series (and poor-quality cohort and case-control study | |
| 5 | Expert opinion without explicit critical appraisal or based on physiology bench research or “first principles” | |
| Grade | Level of Recommendation | Qualifying evidence | Implications for clinicians |
| A | Strong recommendation | Level I evidence or consistent findings from several studies of levels II, III, or IV | These recommendations should be followed unless an alternative approach with clear and compelling rationale is present |
| B | Recommendation | Consistent evidence of levels II, III, or IV | These recommendations should be followed but clinicians should understand that there is scope of improvement and these may be slightly modified as per patient preferences |
| C | Option | Levels II, III, or IV evidence, but findings are inconsistent | These guidelines are broad guidelines and clinicians should be flexible in their decision-making regarding appropriate practice keeping patient preferences in mind |
| D | Option | Level V evidence: Little or no substantial evidence | These recommendations may be referred but clinicians should consider all other available options while making decisions |
Authors (PG, VDY) reviewed all English language articles and tabulated all the evidence available and allotted the level of evidence. After that, the grade of recommendation was decided with consensus of all the authors.
MRI or TRUS are preferred modalities to evaluate Hi-5 fistulas. MRI is better than TRUS to evaluate high secondary extensions. Level of Evidence-2B. Grade of recommendation-B.
MRI and TRUS are the diagnostic modalities of choice to evaluate complex anal fistulas[17,19-21]. MRI and 3D-TRUS have comparable efficacy in outlining the internal opening and primary tracts; however, MRI is significantly more effective than 3D-TRUS in detecting deep secondary extensions[35]. The sensitivity of 3D-TRUS to detect deep secondary extensions was 73.9% while MRI had sensitivity of 97% (P = 0.041)[35]. Therefore, MRI is the preferred modality to diagnose high fistulas especially the high-5 fistulas[10,36].
Due to the challenging factors discussed above, the management of high-5 fistulas is quite difficult and far from satisfactory. Apart from the associated complex parameters, the additional problem is minimal experience or published data on the management of these fistulas. Due to this, the quality of data is of a low evidence level.
As discussed earlier, due to complete involvement of EAS, the most commonly performed procedure, fistulotomy, is contraindicated in these fistulas[25] unless it is coupled with primary sphincter repair. The newer sphincter-sparing procedures, like video-assisted anal fistula treatment[24], fistula laser closure[24], over-the scope clip (OTSC)[37-39], anal fistula plug (AFP)[40], stem cells[28] etc, have dismal success rates in complex fistulas. In fact, there are hardly any studies that have analyzed the success rates of these newer procedures in high-5 fistulas. Therefore, the sphincter-preserving procedures, such as LIFT, FPR, advancement flaps, and TROPIS, seem more suitable procedures for high-5 fistulas.
TROPIS provides a ray of hope, as it has been shown to have satisfactory results in these fistulas[2,3,16,24,41]. In the TROPIS procedure, the intersphincteric space is laid open in the anal canal and the resultant wound is allowed to heal by secondary intention[16,24]. The transsphincteric tracts (tracts lateral to the EAS in the ischiorectal fossa) are thoroughly curetted and cleaned[3,41]. Thus, the tracts on both sides of the EAS are managed separately (tracts inner to the EAS are laid open into the anal canal and tracts outside the EAS in the ischiorectal fossa are curetted and cleaned) and the EAS is not cut or damaged at all[16,24]. Due to this, it has been shown that there is no deterioration in continence after the TROPIS procedure[16,24].
The reason for the higher success rate of the TROPIS procedure could be that, unlike other procedures, TROPIS adequately tackles the sepsis in the intersphincteric space[3,41]. It is now understood that the pus/sepsis in the intersphincteric part of the fistula tract is like an abscess in a closed space[24]. Therefore, this sepsis is best managed in the manner an abscess anywhere else in the body is managed-deroofing the abscess cavity and allowing it to heal by secondary intention[24].
A supralevator abscesses can be drained into the rectum with a moderate success rate[7,42-44]. The level of evidence for this is 2B with a grade of recommendation of B. For example, Garcia-Granero et al[7] drained the supralevator abscess into the rectum in four patients and all of them healed.
This not only leads to resolution of the acute symptoms, but also leads to fistula healing in many cases[7,44].
Fistulectomy with or without advancement flap may be done in selected cases with a moderate success rate[44]. The level of evidence is a -4, with the grade of recommendation a-D. Van Onkelen et al[44] performed advancement flap in three patients after draining the supralevator abscess. All patients healed but multiple surgeries were required in order to achieve fistula healing[44].
Supralevator fistula can be managed with laying open of the supralevator extension into the rectum through the transanal route (TROPIS) procedure with a satisfactory healing rate[6,16]. The level of evidence for this is 2B with a grade of recommendation of B. The success rate was 84.6% (11/13) in the initial series and 82.1% (92/112) on long-term follow-up (median 30 mo) in the same series[16].
LIFT and FPR can also be done in selected cases with good success rates (80%-91%) in high complex fistulas (the studies included supralevator fistulas)[45,46]. The level of evidence for this is 5 with a grade of recommendation of D. However, data for these two procedures specific to supralevator fistulas is not available.
Advancement flap, fistulotomy/fistulectomy with primary sphincter repair and stem cells have moderate success rates, with a success rate range from 70%-85%, though the sample size was quite small[47]. The level of evidence for this is 2B with a grade of recommendation of D. Perez et al[47] compared advancement flap vs fistulotomy with sphincter reconstruction in suprasphincteric fistulas and the healing rate was 80% (4/5) and 83.3% (5/6) respectively[47]. However, the risk of cutting the entire EAS and then repairing the same seems a difficult option to most surgeons. Also, in the rare eventuality of suture dehiscence, the risk of incontinence would be very high.
Fibrin glue has a poor success rate in suprasphincteric fistulas. The level of evidence for this is 2B with a grade of recommendation of C. Garcia-Olmo et al[48] compared the healing rate of fibrin glue vs adipose stem cells in suprasphincteric fistulas. The healing rate in the fibrin glue group was 8% (2/16) while it was 71% (10/14) in the stem cells group[48].
The TROPIS procedure has had promising results with a good success rate with minimal impact on continence The level of evidence for this is 4 with a grade of recommendation of C. Out of 14 suprasphincteric fistulas, 78.6% (11/14) patients were cured with no deterioration in continence levels after surgery on long-term follow-up (median 30 mo)[16].
LIFT can be done in selected cases but is technically difficult. The level of evidence for this is 2B with a grade of recommendation of C.
There are studies in which LFT has shown a good success rate (80%-91%) in high complex fistulas[45,46]. but data for LIFT exclusive to supralevator fistulas is not available. It is also expected that performing the LIFT procedure in suprasphincteric fistulas (ligating the intersphincteric tract high up in the intersphincteric plane) would be a technically demanding procedure.
Temporary colostomy with management of the primary pathology can be used for extrasphincteric fisutlas. The level of evidence for this is 5 with a grade of recommendation of D.
Extrasphincteric fistulas are extremely rare these days, which means there is little data available on the management of these fistulas. As most of these fistulas are caused by secondary pelvic pathology or iatrogenic factors, a diverting stoma along with management of the underlying cause would be the mainstay of treatment[8].
Intra-anal fistulotomy (TROPIS procedure) has a high success rate with minimal impact on continence. The level of evidence for this is 4 with a grade of recommendation of B. These fistulas are perhaps the easiest to treat. Simple intra-rectal fistulotomy (laying open the fistula tract into the anorectum) would cure these fistulas with a high success rate (> 90%)[8,16].
TROPIS has a moderate success rate with minimal impact on continence in RIFIL fistulas. The level of evidence for this is 2B with a grade of recommendation of B. RIFIL fistulas are challenging to manage because access to the RIFIL component might become difficult. TROPIS has shown a moderate success rate in RIFIL fistulas[9]. The healing rate in RIFIL fistulas by the TROPIS procedure was 69.4% (25/36) with a follow-up (median) of 12 mo[9]. There was no negative impact on continence following surgery.
LIFT and FPR are expected to have a moderate success rate; however, since RIFIL fistulas have been recently described[9] and till now, there are no studies of LIFT or FPR procedure in RIFIL fistulas. Conceptually it is expected that these procedures would also prove at least moderately effective for these fistulas. The level of evidence for this is 5 with a grade of recommendation of D.
Anal fistulas that reach up to the levator muscle and involve almost the complete EAS have been grouped together as high-5 fistulas. These comprise supralevator, suprasphincteric, extrasphincteric, high intrarectal, and RIFIL fistulas. The diagnosis as well as the management of these five fistulas is quite challenging. Advanced radiological modalities, preferably MRI, are needed to accurately delineate these fistulas. Once diagnosed, care should be exercised to avoid sphincter-cutting procedures like fistulotomy or cutting seton in these fistulas as the risk of incontinence would be very high. Sphincter-sparing procedures like TROPIS or LIFT should be carried out in these fistulas. These guidelines may help improve understanding and outcomes in the management of these complex fistulas.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Society of Colon Rectum Surgeons; American Society of Gastrointestinal Endoscopic Surgeons; Association of Surgeons of India.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elfeki H, Egypt; Zheng LH, China; Ozkan OF, Turkey; Tamburini AM, Italy S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yu HG
| 1. | Ratto C, Grossi U, Litta F, Di Tanna GL, Parello A, De Simone V, Tozer P, DE Zimmerman D, Maeda Y. Contemporary surgical practice in the management of anal fistula: results from an international survey. Tech Coloproctol. 2019;23:729-741. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Włodarczyk M, Włodarczyk J, Sobolewska-Włodarczyk A, Trzciński R, Dziki Ł, Fichna J. Current concepts in the pathogenesis of cryptoglandular perianal fistula. J Int Med Res. 2021;49:300060520986669. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Li YB, Chen JH, Wang MD, Fu J, Zhou BC, Li DG, Zeng HQ, Pang LM. Transanal Opening of Intersphincteric Space for Fistula-in-Ano. Am Surg. 2021;3134821989048. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Jeong HY, Song SG, Nam WJ, Lee JK. Puborectalis Muscle Involvement on Magnetic Resonance Imaging in Complex Fistula: A New Perspective on Diagnosis and Treatment. Ann Coloproctol. 2021;37:51-57. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Jayne DG, Scholefield J, Tolan D, Gray R, Senapati A, Hulme CT, Sutton AJ, Handley K, Hewitt CA, Kaur M, Magill L; FIAT Trial Collaborative Group. A Multicenter Randomized Controlled Trial Comparing Safety, Efficacy, and Cost-effectiveness of the Surgisis Anal Fistula Plug Versus Surgeon's Preference for Transsphincteric Fistula-in-Ano: The FIAT Trial. Ann Surg. 2021;273:433-441. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Garg P, R Menon G, Kaur B. Comparison of different methods to manage supralevator rectal opening in anal fistulas: A retrospective cohort study. Cir Esp (Engl Ed). 2021;. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Garcia-Granero A, Granero-Castro P, Frasson M, Flor-Lorente B, Carreño O, Espí A, Puchades I, Garcia-Granero E. Management of cryptoglandular supralevator abscesses in the magnetic resonance imaging era: a case series. Int J Colorectal Dis. 2014;29:1557-1564. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1-12. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Garg P, Dawka S, Yagnik VD, Kaur B, Menon GR. Anal fistula at roof of ischiorectal fossa inside levator-ani muscle (RIFIL): a new highly complex anal fistula diagnosed on MRI. Abdom Radiol (NY). 2021;46:5550-5563. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Liu X, Wang Z, Ren H, Ren A, Wang W, Yang X, Shi S. Evaluating postoperative anal fistula prognosis by diffusion-weighted MRI. Eur J Radiol. 2020;132:109294. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Halligan S, Tolan D, Amitai MM, Hoeffel C, Kim SH, Maccioni F, Morrin MM, Mortele KJ, Rafaelsen SR, Rimola J, Schmidt S, Stoker J, Yang J. ESGAR consensus statement on the imaging of fistula-in-ano and other causes of anal sepsis. Eur Radiol. 2020;30:4734-4740. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | De Hous N, Van den Broeck T, de Gheldere C. Fistulectomy and primary sphincteroplasty (FIPS) to prevent keyhole deformity in simple anal fistula: a single-center retrospective cohort study. Acta Chir Belg. 2021;121:308-313. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Ratto C, Litta F, Marra AA, Campennì P, De Simone V, Parello A. Fistulotomy plus end-to-end primary sphincteroplasty - a video vignette. Colorectal Dis. 2021;23:2213-2214. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Litta F, Parello A, Ferri L, Torrecilla NO, Marra AA, Orefice R, De Simone V, Campennì P, Goglia M, Ratto C. Simple fistula-in-ano: is it all simple? Tech Coloproctol. 2021;25:385-399. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Fröhlich B, Hötzinger H, Fritsch H. Tomographical anatomy of the pelvis, pelvic floor, and related structures. Clin Anat. 1997;10:223-230. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Garg P, Kaur B, Goyal A, Yagnik VD, Dawka S, Menon GR. Lessons learned from an audit of 1250 anal fistula patients operated at a single center: A retrospective review. World J Gastrointest Surg. 2021;13:340-354. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | van Rijn KL, Lansdorp CA, Tielbeek JAW, Nio CY, Buskens CJ, D'Haens GRAM, Löwenberg M, Stoker J. Evaluation of the modified Van Assche index for assessing response to anti-TNF therapy with MRI in perianal fistulizing Crohn's disease. Clin Imaging. 2020;59:179-187. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Balcı S, Onur MR, Karaosmanoğlu AD, Karçaaltıncaba M, Akata D, Konan A, Özmen MN. MRI evaluation of anal and perianal diseases. Diagn Interv Radiol. 2019;25:21-27. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Konan A, Onur MR, Özmen MN. The contribution of preoperative MRI to the surgical management of anal fistulas. Diagn Interv Radiol. 2018;24:321-327. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Almeida IS, Jayarajah U, Wickramasinghe DP, Samarasekera DN. Value of three-dimensional endoanal ultrasound scan (3D-EAUS) in preoperative assessment of fistula-in-ano. BMC Res Notes. 2019;12:66. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Youssef A. The Ultrasound of Perianal External Opening. Gastroenterol Hepatol Int J. 2017;2:000128. [Cited in This Article: ] |
| 22. | Lefrançois P, Zummo-Soucy M, Olivié D, Billiard JS, Gilbert G, Garel J, Visée E, Manchec P, Tang A. Diagnostic performance of intravoxel incoherent motion diffusion-weighted imaging and dynamic contrast-enhanced MRI for assessment of anal fistula activity. PLoS One. 2018;13:e0191822. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Erden A. MRI of anal canal: normal anatomy, imaging protocol, and perianal fistulas: Part 1. Abdom Radiol (NY). 2018;43:1334-1352. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Garg P, Sodhi SS, Garg N. Management of Complex Cryptoglandular Anal Fistula: Challenges and Solutions. Clin Exp Gastroenterol. 2020;13:555-567. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Nottingham JM, Rentea RM. Anal Fistulotomy. StatPearls. Treasure Island (FL), 2021. [Cited in This Article: ] |
| 26. | Buhulaigah H, Truong A, Zaghiyan K, Fleshner P. Clinical Factors Associated With Response to Fecal Diversion in Crohn's Disease. Am Surg. 2020;86:1277-1280. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Truong A, Zaghiyan K, Fleshner P. Anorectal Crohn's Disease. Surg Clin North Am. 2019;99:1151-1162. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Cao Y, Su Q, Zhang B, Shen F, Li S. Efficacy of stem cells therapy for Crohn's fistula: a meta-analysis and systematic review. Stem Cell Res Ther. 2021;12:32. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Garg P, Goyal A, Yagnik VD, Dawka S, Menon GR. Diagnosis of anorectal tuberculosis by polymerase chain reaction, GeneXpert and histopathology in 1336 samples in 776 anal fistula patients. World J Gastrointest Surg. 2021;13:355-365. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Guyatt G, Gutterman D, Baumann MH, Addrizzo-Harris D, Hylek EM, Phillips B, Raskob G, Lewis SZ, Schünemann H. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an american college of chest physicians task force. Chest. 2006;129:174-181. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Aguayo-Albasini JL, Flores-Pastor B, Soria-Aledo V. [GRADE system: classification of quality of evidence and strength of recommendation]. Cir Esp. 2014;92:82-88. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Chen Y, Yang K, Marušic A, Qaseem A, Meerpohl JJ, Flottorp S, Akl EA, Schünemann HJ, Chan ES, Falck-Ytter Y, Ahmed F, Barber S, Chen C, Zhang M, Xu B, Tian J, Song F, Shang H, Tang K, Wang Q, Norris SL; RIGHT (Reporting Items for Practice Guidelines in Healthcare) Working Group. A Reporting Tool for Practice Guidelines in Health Care: The RIGHT Statement. Ann Intern Med. 2017;166:128-132. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Appraisal of Guidelines for Research and Evaluation. Global Rating Scale. [cited 16 September 2021]. Available from: https://www.agreetrust.org/wp-content/uploads/2017/11/AGREE-GRS.pdf. [Cited in This Article: ] |
| 34. | Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128:305-310. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Brillantino A, Iacobellis F, Reginelli A, Monaco L, Sodano B, Tufano G, Tufano A, Maglio M, De Palma M, Di Martino N, Renzi A, Grassi R. Preoperative assessment of simple and complex anorectal fistulas: Tridimensional endoanal ultrasound? Radiol Med. 2019;124:339-349. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Mathew RP, Patel V, Low G. Caution in using 3D-EAUS as the first-line diagnostic tool in the preoperative work up for perianal fistulas. Radiol Med. 2020;125:155-156. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Grossberg SJ, Harran N, Bebington B, Lutrin DL. Use of the OVESCO OTSC® Proctology Clip for closure of fistula-in-ano at Wits Donald Gordon Medical Centre - a single centre experience. S Afr J Surg. 2020;58:74-77. [PubMed] [Cited in This Article: ] |
| 38. | Mascagni D, Pironi D, Grimaldi G, Romani AM, La Torre G, Eberspacher C, Palma R, Sorrenti S, Pontone S. OTSC® Proctology vs. fistulectomy and primary sphincter reconstruction as a treatment for low trans-sphincteric anal fistula in a randomized controlled pilot trial. Minerva Chir. 2019;74:1-6. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Marinello F, Kraft M, Ridaura N, Vallribera F, Espín E. Treatment of Fistula-in-ano With OTSC® Proctology Clip Device: Short-term Results. Cir Esp (Engl Ed). 2018;96:369-374. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Tao Y, Zheng Y, Han JG, Wang ZJ, Cui JJ, Zhao BC, Yang XQ. Effects of an anal fistula plug on anal function after surgery for treatment of a trans-sphincteric anal fistula. Langenbecks Arch Surg. 2021;406:855-861. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Huang B, Wang X, Zhou D, Chen S, Li B, Wang Y, Tai J. Treating highly complex anal fistula with a new method of combined intraoperative endoanal ultrasonography (IOEAUS) and transanal opening of intersphincteric space (TROPIS). Wideochir Inne Tech Maloinwazyjne. 2021;16:697-703. [PubMed] [DOI] [Cited in This Article: ] |
| 42. | Bevans DW Jr, Westbrook KC, Thompson BW, Caldwell JT. Perirectal abscess: a potentically fatal illness. Am J Surg. 1973;126:765-768. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Ferguson EF Jr, Houston CH. Iatrogenic supralevator fistula. South Med J. 1978;71:490-495. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | van Onkelen RS, Gosselink MP, Schouten WR. Treatment of anal fistulas with high intersphincteric extension. Dis Colon Rectum. 2013;56:987-991. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Malakorn S, Sammour T, Khomvilai S, Chowchankit I, Gunarasa S, Kanjanasilp P, Thiptanakij C, Rojanasakul A. Ligation of Intersphincteric Fistula Tract for Fistula in Ano: Lessons Learned From a Decade of Experience. Dis Colon Rectum. 2017;60:1065-1070. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Litta F, Parello A, De Simone V, Grossi U, Orefice R, Ratto C. Fistulotomy and primary sphincteroplasty for anal fistula: long-term data on continence and patient satisfaction. Tech Coloproctol. 2019;23:993-1001. [PubMed] [DOI] [Cited in This Article: ] |
| 47. | Perez F, Arroyo A, Serrano P, Sánchez A, Candela F, Perez MT, Calpena R. Randomized clinical and manometric study of advancement flap vs fistulotomy with sphincter reconstruction in the management of complex fistula-in-ano. Am J Surg. 2006;192:34-40. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79-86. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | CEBM. OCEBM levels of evidence. [cited 11 January 2022]. Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. [Cited in This Article: ] |