Abstract
Copper ferrite, belonging to the wide and technologically relevant class of spinel ferrites, was grown in the form of t-CuFe2O4 nanocrystals within a porous matrix of silica in the form of either an aerogel or a xerogel, and compared to a bulk sample. Extended X-ray absorption fine structure (EXAFS) spectroscopy revealed the presence of two different sub-lattices within the crystal structure of t-CuFe2O4, one tetragonal and one cubic, defined by the Cu2+ and Fe3+ ions respectively. Our investigation provides evidence that the Jahn-Teller distortion, which occurs on the Cu2+ ions located in octahedral sites, does not affect the coordination geometry of the Fe3+ ions, regardless of their location in octahedral or tetrahedral sites.
Similar content being viewed by others
Introduction
Copper ferrite is a ceramic material belonging to a class of metal-oxides of generic formula MFe2O4 where M represents a bi-valent transition metal ion, e.g. Mn2+, Ni2+, Co2+, Cu2+ 1, possessing the spinel structure.
Spinel ferrites in form of nanoparticles have received increasing attention mainly because of their magnetic and catalytic properties2 which are different from their parent bulk3 and are dependent on the size of the nanoparticles and the cation distribution within the structure4,5,6,7. In particular, copper ferrite nanoparticles have been shown to be suitable candidates for a wide range of technologically important applications, such as high energy storage materials8,9, magnetically recoverable catalysts10 for photocatalytic water splitting11 and as gas sensors12.
Many methods for the synthesis of copper ferrite nanoparticles have been proposed so far, the most common include high temperature solution phase approaches13, thermal decomposition of nitrates14, co-precipitation15, combustion process16 and sol-gel auto-combustion17,18.
However, these methods yield nanoparticles with average dimensions of several tens of nanometres and a poor degree of size and shape homogeneity. This is likely due to the aggregation and coalescence of the nanoparticles under the required thermal treatments. As an example, the sol-gel autocombustion process proposed by Ansari et al. for the synthesis of lead and copper hexaferrite (PbFe12O19 and CuFe12O19 respectively) using maltose as reducing agent19,20 enables to tune the size and shape of the produced nanoparticles by control of the calcination temperatures and the maltose content. Spherical nanoparticles with sizes of about 20 nm were obtained, but their TEM characterization clearly shows their tendency to agglomerate, which is a typical problem encountered for unsupported nanoparticles.
On the other hand, the control of the particle size has a major technological importance, because the magnetic and catalytic properties of nanoparticles are highly size dependent16.
The confined crystallisation of nanoparticles inside an amorphous matrix such as silica is a successful method for achieving the control of crystal growth while at the same time avoiding coalescence and growth. Previously, our group synthesised nanocomposites where cubic Mn, Ni, Co and Zn ferrite nanoparticles with average dimensions ranging from 5 to 10 nm are dispersed within the highly porous matrix of a silica aerogel21,22. In these nanocomposites, the nanoparticles are kept apart by the very highly porous silica matrix, avoiding their aggregation under thermal treatments, yielding nanoparticles with a high degree of size and shape homogeneity.
The ability to control the growth of ferrite nanoparticles has been used in this paper to investigate if the crystalline structure of copper ferrite is different at the nanoscale with respect to the bulk and if it is influenced by the presence of a silica matrix of variable density and texture. In particular, copper ferrite nanocrystals have been grown by confined crystallization in either a very low density silica aerogel matrix or a denser xerogel matrix.
Most of spinels have a cubic unit cell belonging to the Fd-3m space group, in which 24 metal ions are distributed in 8 tetrahedral and 16 octahedral sites within the close-packed arrangement made up of 32 oxygen ions. When the tetrahedral sites are entirely occupied by the bi-valent cation, the spinel is referred to as ‘normal’. When the bi-valent cations are located only in octahedral sites, the spinel is called ‘inverse’. Identifying with A and B the tetrahedral and octahedral sites respectively, the generic formula of a ferrite can be written as follows23:
with ‘i’ the inversion degree of the spinel and α the concentration of oxygen vacancies, if any. Many spinels undergo distortion upon ordering at low temperature, with the formation of a tetragonal I4I/amd unit cell.
Bulk copper ferrite is reported in the literature as an inverse spinel (i ≈ 1), which crystallises in either the tetragonal I4I/amd phase or the Fd-3m cubic one24. The tetragonal copper ferrite (t-CuFe2O4) is stable at room temperature and a transition to the cubic phase (c-CuFe2O4) is observed around 360–420 °C, where the two phases coexist, with a minor change in the inversion degree16,25. It was found that the tetragonal to cubic phase transition is accompanied by the reduction of part of Cu2+ to Cu+ ions, forming an oxygen deficient copper ferrite26,27. This evidence is supported by the possibility to selectively synthesise c-CuFe2O4 by performing a high temperature thermal treatment and the subsequent cooling process under inert atmosphere28.
In copper ferrite the tetragonal phase arises from the distortion along one of the axis of the octahedrons, caused by the presence of Cu2+ ions (d9), which impose the typical Jahn-Teller distortion, by the removal of the eg orbital degeneracy29,30. Because the copper and iron cations share the octahedral sites in the spinel structure of the t-CuFe2O4, the unit cell is usually described with the Fe3+ ions having the same distortion, although there is no crystal-field stabilisation energy associated with the distortion at the Fe3+ (d5) sub-lattice.
In the literature, a considerable amount of work is found on the crystal structure of copper ferrite, where techniques such as X-ray diffraction (XRD), neutron diffraction and Mössbauer spectroscopy were mainly used5,24,25. However, diffraction techniques provide only an average structure and while Mössbauer spectroscopy can be used to study the inversion degree in the spinel structures31, it is not useful to study the copper environment that is essential to study the distortion produced in the tetragonal phase of copper ferrite by the presence of the Cu2+ ions.
X-ray absorption fine structure (XAFS) has the ability to study selectively and independently the Cu2+ and Fe3+ sites and has demonstrated to be exceptionally successful in studying ferrite structures32,33,34,35,36,37 as XAFS is element-specific and sensitive to the coordination environment of each specific absorbing atom. In the case of copper ferrite, the use of XAFS has been so far quite limited with the most comprehensive study being by Krishnan et al.38 where a structural investigation on CuFe2O4 nanoparticles was performed. Their Extended X-ray Absorption Fine Structure (EXAFS) and X-ray Absorption Near Edge Structure (XANES) results revealed that the cation distribution of copper ferrite nanoparticles is analogue to that of a bulk sample and all the Cu2+ ions occupy octahedral positions (complete inverse spinel). However, limited structural information was achieved on the local environment of Fe3+ ions since their EXAFS fit at the Fe K-edge did not distinguish between ions located in octahedral and tetrahedral sites.
In this paper XAFS has been used to study in detail the structure of CuFe2O4 in a bulk sample and in two nanocomposites where CuFe2O4 nanoparticles have been grown in either a very low density silica aerogel matrix or a denser xerogel matrix. The results clearly evidence for the first time that the accepted crystalline structure for t-CuFe2O4 based on diffraction methods lacks specific details, with the Jahn-Teller distortion in reality only affecting the copper ions.
Methods
Synthesis of the CuFe2O4 − SiO2 nanocomposites
Sol-gel procedure
The synthesis of the CuFe2O4 − SiO2 nanocomposites has been carried out following a 2-step catalysed sol-gel procedure previously published by our group22, which was successful in producing silica-based multicomponent gels containing a metal ferrite as dispersed phase, leading to high-quality MFe2O4 − SiO2 aerogels, where M = Mn, Ni, Co, Zn21,31. In this paper this synthetic protocol was used for the first time to produce both an aerogel and a xerogel nanocomposite with the same composition.
Briefly, in the first step an ethanolic solution of the metal salts (0.2299 g, 9.69∙10−4 mol, of Cu(NO3)2∙2.5H2O, Aldrich, 98%, 0.7985 g, 1.94∙10−3 mol, of Fe(NO3)3∙9H2O, Aldrich, 98%, 7.5 mL of absolute ethanol, Fisher Chemicals) was added to the pre-hydrolysed TEOS (7.9 mL, Aldrich, 98%) under acidic catalysis. The amounts of the precursors were calculated in such a way to produce a nanocomposite containing 10 wt.% CuFe2O4/(CuFe2O4 + SiO2). A solution containing urea (3.513 g of urea, NH2CONH2 Aldrich, >99%, 9 mL of absolute ethanol, Fisher Chemicals, 4.92 mL of distilled water) was then added under reflux for 128′ at 85 °C, in the second step to modulate pH achieving a fast gelation but at the same time avoiding the precipitation of metal hydroxides. The obtained sol was poured in cylindrical vials, which were sealed and kept at 40 °C until gelation, which took place within 20 h.
Aerogel nanocomposite
In order to obtain a CuFe2O4 − SiO2 aerogel, the solvent was removed from the alcogel in the supercritical state. To do so, the alcogel was inserted in a Parr 300 mL stainless-steel autoclave together with 70 mL of absolute ethanol. The autoclave was sealed and flushed with pure N2 to ensure an inert atmosphere. The autoclave was heated up to a temperature of 330 °C when the corresponding pressure of 70 atm was achieved, ensuring that the solvent was in the supercritical state. The autoclave was then slowly vented at constant temperature and the aerogel was obtained.
Xerogel nanocomposite
A CuFe2O4 − SiO2 xerogel was obtained by submitting the alcogel to slow evaporation of the solvent in an open container, at 40 °C for 70 h until constant weight.
Thermal treatments
After drying, the aerogel and xerogel samples were powdered and submitted to the same calcination treatments, performed in static air. The dried gels were calcined first at 450 °C for 1 h, to remove organics, and then at 750 °C for 1 h, 750 °C for 6 h and 900 °C for 1 h to promote the growth of the copper ferrite nanoparticles. The formation of the CuFe2O4 is complete only under thermal treatment at 900 °C, as previously observed in other ferrite-silica nanocomposites21,22,31,33,35,36. The samples will be named hereafter as ACuFe and XCuFe to indicate the aerogel and xerogel respectively.
Synthesis of bulk – CuFe2O4
The synthesis of a bulk sample of CuFe2O4 was performed following a procedure already reported in the literature39. According to this procedure, appropriate amounts of metal nitrates were dissolved in water and the solution was heated at 95 °C up to dryness. The resulting solid was calcined first at 150 °C, allowing for the complete decomposition of nitrates. Further calcination at 900 °C for 8 h followed by slow cooling in static air is needed to promote the formation of tetragonal copper ferrite. The sample will be named hereafter as bulk–CuFe2O4.
Characterization of the nanocomposites
X-ray Diffraction (XRD) patterns were recorded on a Panalytical Empyrean diffractometer equipped with a graphite monochromator on the diffracted beam and a X’Celerator linear detector. The scans were collected within the range of 10–90° (2θ) using Cu Kα radiation. The average size of crystallite domains was calculated using the Scherrer equation, t = 0.91 λ/(B cos θ), where t is the crystallite size, λ is the incident radiation wave-length, θ is the Bragg angle and B is the full-width at half-maximum of the diffraction peak, corrected for instrumental broadening using a standard LaB6 sample40.
Transmission electron microscopy (TEM) images were recorded on a Hitachi H-7000 instrument running at 125 kV and equipped with an AMT DVC (2048 × 2048 pixel) CCD Camera. Prior to observation, the samples were finely ground and deposited on a carbon-coated copper grid. SEM images were recorded on a Hitachi S4700 FEG-SEM.
The X-ray absorption spectra were recorded on the B18 beamline at the DIAMOND synchrotron (Oxfordshire, UK). XANES and EXAFS spectra at the Fe (7112 eV) and Cu (9790 eV) K-edge were collected at room temperature in transmission mode using a Si(111) monochromator. The monochromator energy scale was calibrated via a third ion chamber with Fe/Cu foils. The stability of the energy scale was ±0.1 eV. The samples, in form of powder, were diluted with polyvinylpyrrolidone (PVP) in an appropriate concentration and pressed to form a pellet. The data analysis was performed using the ATHENA and ARTEMIS software41. With ATHENA, the absorption edge, E0, is determined, and the absorption due to the isolated atom is subtracted, by fitting the pre-edge and post-edge regions to obtain χ(k). ATHENA is also used to process the XANES data. The software ARTEMIS is used to perform the fit of the EXAFS region to scattering models in R-space obtained by FEFF, validated on standard compounds. The number of fitted parameters was always lower than the number of independent points.
Results and Discussion
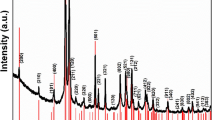
In Fig. 1, the XRD patterns of the ACuFe and XCuFe are shown, together with the bulk sample. The reference patterns of the tetragonal and cubic CuFe2O4 crystalline phases are also shown as a refs42,43. The pattern of the bulk sample can be unambiguously identified as corresponding to the tetragonal phase. In fact, the peaks at 2θ values of ~31°, 34.5° and 41.4°, found in the XRD pattern of the bulk sample, can only be assigned to the tetragonal phase. Likewise, some peaks corresponding only to the cubic phase, such as one at 2θ = 43.2° were not found in the XRD pattern of the bulk-CuFe2O4.
XRD patterns of ACuFe, XCuFe and bulk-CuFe2O4. The pattern of the tetragonal and cubic phases of copper ferrite are also reported. *Represents a peak identified as a small impurity of CuO48.
The patterns of the aerogel and xerogel samples show the typical halo due to the amorphous silica matrix and the superimposed broad peaks corresponding to nanocrystalline copper ferrite. Due to the broadening of the peaks, it is not possible to determine if the copper ferrite nanoparticles are in the tetragonal or cubic crystalline phase. The average sizes of the crystallites, determined applying the Scherrer equation to the most intense peak at ∼35° and correcting for instrumental broadening, are 6 nm in the case of the aerogel and 7 nm in the case of the xerogel sample. The typical error on the determination of the average crystal size is ± 1 nm.
The XRD characterization of the nanocomposites, as a function of the calcination temperature, is reported in Figures S1 and S2, Supp. Info. The XRD patterns show that the peaks corresponding to the CuFe2O4 crystalline phase appear only upon calcination at 750 °C for 1 h. However, the formation of the CuFe2O4 phase is only complete after a thermal treatment of 900 °C as inferred by inspecting the relative intensity of the peaks. These results are also in agreement with previous findings in other ferrite-silica nanocomposites21,22,31,33,35,36.
In Fig. 2, bright field and dark field images of the aerogel and xerogel samples are shown. The TEM images in bright field mode shows the highly porous texture typical of the aerogel, Fig. 2(a), while the xerogel sample appears denser and less porous, as expected, Fig. 2(c). This difference in porosity has also been confirmed by N2-physisorption reported in the SI, with the xerogel having a much lower pore volume and reduced surface area with respect to the aerogel. By looking at the dark field TEM images in Fig. 2(b) and (d), the copper ferrite dispersed nanophase can be seen as bright spots on top of the darker background due to the silica matrix. The nanoparticles appear uniformly dispersed within the porous matrix. In the case of the xerogel (Fig. 2(d)), due to the denser silica matrix, the nanocrystallites are only visible on the edges of the particles. The average size of the nanocrystals is in agreement with the values determined from the XRD patterns, confirming that the Scherrer equation was able to provide the correct value. The size of the crystals is similar in the two composites despite their very different texture and porosity, indicating the growth of nanocrystals within the matrix is driven by the thermal treatment of the gels.
FESEM investigation was used to gain insights on the texture and compositional homogeneity of the materials. The images reported in Fig. 3 for the aerogel, xerogel and bulk samples support the results obtained by TEM and physisorption investigation. In particular, the highly porous texture of the aerogel, the denser features in the xerogel due to the occurrence of smaller pores and the microcrystalline aspect of the bulk ferrite sample are observed.
Symmetry and coordination at the metal sites
XAFS spectroscopy has been used for the characterization of the ACuFe, XCuFe and the bulk sample. The analysis of the XANES region gives information on the oxidation state of the absorbing atom and on its coordination geometry. In Fig. 4, the XANES spectra of the samples are reported, at both the Cu and Fe K-edges. At the Fe K-edge, the spectrum of γ-Fe2O3 (maghemite) reference compound is reported superimposed to those of the samples while, at the Cu K-edge, the absorption spectrum of CuO (tenorite) reference compound is reported.
In Table 1 the absorption edges (E0) and the corresponding oxidation states are reported. By comparing the position of the absorption edges with reference compounds the oxidation states are Fe3+ and Cu2+ for all samples37.
A close inspection of the pre-edge region, enlarged in the insets of Fig. 4, provides qualitative information on the coordination geometry of the absorbing atom. In this region of the spectra, a pre-peak might be present, due to electronic transition involving the 3d orbitals44. The intensity of the peak is sensitive to the local environment of the absorbing atom, and is more intense in the presence of non-centrosymmetric coordination geometry, e.g. when the cation is located in a tetrahedral site, as in γ-Fe3O3. A well-defined pre-peak is present in the case of the Fe K-edge for both the nanocomposites and for the bulk sample, which is indicative of the presence of Fe3+ cations in tetrahedral sites. In the case of the Cu K-edge, the pre-peak has a very low intensity, in both CuO and CuFe2O4. Note that Cu2+ has a d9 configuration and the pre-edge peak is due to 1s-3d transitions with an overwhelming 1sd9 configuration45. As in other transition metal K-edges, the Cu pre-edge peak intensity is sensitive to the degree of centrosymmetry of the Cu site46, and is significantly larger for tetrahedral coordination (e.g. in CuAl2O4 and CuCr2O4) and smaller for octahedral coordation (e.g. CuO and CuFe2O4)47. The similar, low pre-edge peak intensities observed for CuO and for the CuFe2O4 samples in Fig. 4 suggest that all the Cu2+ cations are located in octahedral sites. The analysis of the pre-peak region therefore suggests that the spinel is completely (or highly) inverted, as expected for CuFe2O4. We also note that the pre-edge and post-edge features of the xerogel and aerogel samples are very similar to those of the bulk, which suggests a close similarity of the iron and copper ions environment in the two nanocomposites and the bulk.
Distortions at the Fe and Cu sub-lattices
The analysis of the EXAFS region at both the Cu and Fe K-edges allows obtaining in depth and quantitative insights on the coordination environment of each metal ion within the crystal structure, including the distances of each coordination shell. In Fig. 5, the k3χ(k) and the corresponding Fourier Transforms (FTs) are reported for the nanocomposites and the bulk sample at the Cu K-edge. The same is reported for the Fe K-edge in Fig. 6.
First of all, the qualitative analysis of the FTs can give further confirmation regarding the inversion degree of the spinel. At both the Cu and Fe K-edges, the first peak is generated by the distances between the absorbing atom and the first coordination shell of oxygen atoms. The second peak is mainly due to distances between the absorber with either copper or iron. In particular, two main unresolved contributions can be identified: one centred around 2.9 Å and the second centred around 3.5 Å. In the spinel structure, 2.9 Å corresponds to the distance between two octahedral sites (MB-MB) while the distances involving tetrahedral sites (MB-MA, MA-MB and MA-MA) are all centred at about 3.5 Å. By looking at the FTs at the Cu K-edge, Fig. 5, the peak centred at 2.9 has the highest intensity suggesting that the majority of the Cu2+ ions are located in octahedral sites. The much less intense peak around 3.5 Å is therefore likely due to distances between the Cu2+ absorbing ions with Fe3+ backscattering ions, located in tetrahedral sites. The same features were found in a previous work, in the case of Ni ferrite, which is an inverse spinel32. In the case of the Fe K-edge, the iron ions seem to be equally distributed between octahedral and tetrahedral sites, since the contribution at 2.9 Å due to MB-MB distances and the one at 3.5 Å due to MA-MB, MB-MA and MA-MA distances have comparable heights.
Quantitative information was obtained performing the fitting of the EXAFS region, after carefully choosing the theoretical model. In all the best fitting only single scattering paths were added after checking that the contribution of multiple scattering paths was less significant. A first attempt in fitting the data at the Cu K-edge employing a model based on the cubic copper ferrite crystalline phase did not give satisfactory results on neither of the samples. This was not surprising in the case of the bulk sample, as XRD clearly shows that the bulk sample is in the tetragonal phase and the cubic model does not take into account the Jahn-Teller distortion, whose effect is strongest on the first coordination shell.
A good fit of the EXAFS region at the Cu K-edge was instead obtained for the bulk sample and for the nanocomposites using the same theoretical model based on the tetragonal copper ferrite crystalline phase, with inversion degree i = 1, which takes into account the effect of the Jahn-Teller distortion. Nine coordination shells, up to a distance of 4 Å, were included in the fitting. Coordination numbers were kept fixed, given by the crystal structure, while the distances (ΔRi), the Debye-Waller factors (σ2) and ΔE0 were left free to vary. S02 was determined by fitting the data of the bulk sample, and then kept fixed for the nanocrystalline samples. The crystal structure of tetragonal copper ferrite requires that the MB-MB distances are split in two contributions, since the octahedral sites are occupied by both Cu2+ and Fe3+ ions with 50% occupancy. However, this does not increase the number of fitted parameters because the same distances (ΔRi) and Debye-Waller factors (σ2) are imposed throughout the fitting. The Cu-O distances at R > 3.5 Å and the corresponding Debye Waller factors were kept fixed, due to their small weight. The results of the fit are very similar for all the samples, indicating that the copper environment is typical of the tetragonal phase also for the xerogel and aerogel samples, where the XRD results where ambiguous, due to peak broadening. Best fit parameters are shown in Table 2 for the aerogel nanocomposite and those for the bulk and the xerogel samples are reported in Tables S2 and S3 in the SI.
When performing the fit of the EXAFS region at the Fe K-edge the same theoretical model corresponding to the tetragonal phase which was used for fitting the Cu K-edge, did not produce satisfactory results. The best fit was obtained instead using the model of the cubic copper ferrite crystalline phase, keeping the inversion degree i = 1. Because of the inversion the Fe3+ absorbing ions must be split into two series of contributions, scaled to 50% occupancy, the first involving Fe3+ in the octahedral sites and the second involving the Fe3+ in tetrahedral sites respectively. Distances up to 4 Å were fitted, which includes 4 shells to fit the contributions due to Fe3+ in the tetrahedral sites and 5 shells to fit the contributions due to Fe3+ in the octahedral sites. Because of the crystal structure of cubic copper ferrite, several paths need to be split in the fitting. However, this does not overcomplicate the fitting because the same distances (ΔRi) and Debye-Waller factors (σ2) are imposed throughout the fitting for any distance which is the same from a crystallographic point of view. In particular, the distances with backscattering ions in octahedral sites (MA-MB and MA-MB) need to be split because they are involving both Fe3+ and Cu2+, scaling the occupancy of each contribution by a further 50% while imposing the same distances (ΔRi) and Debye-Waller factors (σ2). Moreover, the FeA-FeB and FeB-FeA contributions represent the same distance and therefore the additional constraint of using the same ΔRi and σ2 was used. Finally, the same ΔRi and σ2 were used to fit two distances at 3.6 Å (FeA-O and FeB-O), since they are too close to be distinguished by EXAFS, even if they are different from a crystallographic point of view.
Also in the case of the Fe K-edge the results of the fit are very similar for all samples. The best fit parameters are shown in Table 3 for the aerogel nanocomposite and those for the bulk and xerogel samples are reported in Tables S4 and S5 in the SI.
The cooperative Jahn-Teller distortion, occurring at the octahedral sites occupied by the Cu2+ ions, causes the formation of the tetragonal phase. The fit of the EXAFS region at the Cu K-edge confirms that the six Cu-O distances are splitted into 4 short Cu-O distances and two longer ones. However, the octahedral sites within the unit cell are 50% occupied by Cu2+ ions, with Fe3+ ions occupying the other 50% of the sites. By only using XRD it is not possible to discriminate whether the octahedral sites occupied by the Fe3+ ions are also affected by this change in symmetry. This is because XRD provides the average structural environment, and as a consequence the published crystal structures for tetragonal copper ferrite is not distinguishing between the two cations24. Instead, EXAFS spectroscopy allowed us to find clear evidence that all the six Fe-O distances in the first coordination shell of copper ferrite are identical (see Table 3) since a good fit at the Fe K-edge was only obtained by using a model based on the cubic crystalline phase. It should be noted that also when fitting only the first peak in the FTs the same results were obtained, i.e. best fit was obtained using the tetragonal copper ferrite phase at the Cu K-edge and the cubic copper ferrite phase at the Fe K-edge.
These results therefore unambiguously indicate that the Fe3+ ions define a cubic sub-lattice within the tetragonal lattice defined by the Cu2+ ions. This is likely possible because of the presence of a number of available interstitial sites within the spinel crystal structure, where the Fe3+ ions can migrate in order to avoid any axial distortion on their first coordination shell.
Conclusions
The synthesis of t-CuFe2O4 nanocrystals with controlled features has been achieved by performing the crystal growth within the amorphous porous matrix of a silica aerogel and xerogel. The structural and textural features as assessed by TEM, XRD and N2-physisorption point out to the formation of t-CuFe2O4 nanophase with crystals sizes in the region of 6–7 nm and high size homogeneity, both in a relatively dense xerogel and in a highly porous aerogel.
EXAFS and XANES investigation carried out at the Fe and Cu K-edges indicates that the structure of the nanocrystalline samples is very similar to that of the bulk t-CuFe2O4. Very importantly, EXAFS spectroscopy has shed a new perspective on the crystalline structure of tetragonal copper ferrite, which is retained at the nanophase. In particular, two different sub-lattices are present within the structure, one tetragonal and one cubic, defined by Cu2+ and Fe3+ ions respectively. This is ascribed to the difference in the coordination environment of the two different cations located in the octahedral sites. In particular, the electronic structure of Cu2+ (d9) imposes the axial distortion of the octahedron, i.e. the Jahn-Teller distortion, while the coordination environment of the Fe3+ ions located in the remaining half of the octahedral sites remains unaffected and can therefore be regarded as a cubic sub-lattice. This demonstrates that the ability of studying the Cu and Fe local environment separately and independently is able to emphasize local structural features that diffraction techniques cannot achieve since they provide an average structure.
We believe that a detailed description of the oxidation state and coordination symmetry at the metal sites could provide a deeper understanding of the properties of copper ferrite and copper-based nanoparticles which have prospective technologically important applications as cost-effective non noble metal catalysts, high energy storage materials, and sensors.
References
Kefeni, K. K., Msagati, T. A. & Mamba, B. B. Ferrite nanoparticles: synthesis, characterisation and applications in electronic device. Mater. Sci. Eng.: B 215, 37–55 (2017).
Wang, D. & Astruc, D. Fast-growing field of magnetically recyclable nanocatalysts. Chem. Rev. 114, 6949–6985 (2014).
Qu, Y. et al. The effect of reaction temperature on the particle size, structure and magnetic properties of co-precipitated CoFe2O4 nanoparticles. Mater. Lett. 60, 3548–3552 (2006).
Tang, Z. X., Sorensen, C. M., Klabunde, K. J. & Hadjipanayis, G. C. Size-dependent Curie temperature in nanoscale MnFe2O4 particles. Phys. Rev. Lett. 67(25), 3602 (1991).
Tiano, A. L. et al. Correlating size and composition-dependent effects with magnetic, Mössbauer, and pair distribution function measurements in a family of catalytically active ferrite nanoparticles. Chem. Mater. 27, 3572–3592 (2015).
Gore, S. K. et al. The structural and magnetic properties of dual phase cobalt ferrite. Sci. Rep. 7, 2524 (2017).
Wei, G. et al. Reversible control of the magnetization of spinel ferrites based electrodes by lithium-ion migration. Sci. Rep. 7, 12554 (2017).
Cama, C. A. et al. Redox chemistry of a binary transition metal oxide (AB2O4): a study of the Cu2+/Cu0 and Fe3+/Fe0 interconversions observed upon lithiation in a CuFe2O4 battery using X-ray absorption spectroscopy. Phys. Chem. Chem. Phys. 18(25), 16930–16940 (2016).
Wang, J. et al. Copper ferrites@reduced graphene oxide anode materials for advanced lithium storage applications. Sci. Rep. 7, 8903 (2017).
Wang, Z. L. Magnetically separable CuFe2O4 nanoparticles as a recoverable catalyst for the addition reaction of C (sp 3)–H bond of azaarenes to aldehydes. RSC Adv. 5(8), 5563–5566 (2015).
Díez‐García, M. I., Lana‐Villarreal, T. & Gómez, R. Study of copper ferrite as a novel photocathode for water reduction: improving its photoactivity by electrochemical pretreatment. ChemSusChem 9(12), 1504–1512 (2016).
Haija, M. A., Abu-Hani, A. F., Hamdan, N., Stephen, S. & Ayesh, A. I. Characterization of H2S gas sensor based on CuFe2O4 nanoparticles. J. Alloy Compd. 690, 461–468 (2017).
Lee, J., Zhang, S. & Sun, S. High-temperature solution-phase syntheses of metal-oxide nanocrystals. Chem. Mater. 25, 1293–11304 (2013).
Stoia, M., Barvinschi, P. & Barbu-Tudoran, L. Thermal decomposition of metal nitrates. J. Therm. Anal. Calorim. 113(1), 21–30 (2013).
Salavati-Niasari, M. et al. Synthesis and characterization of copper ferrite nanocrystals via coprecipitation. J. Clust. Sci. 23(4), 1003–1010 (2012).
Köferstein, R., Walther, T., Hesse, D. & Ebbinghaus, S. G. Crystallite-growth, phase transition, magnetic properties, and sintering behaviour of nano-CuFe2O4 powders prepared by a combustion-like process. J. Solid State Chem. 213, 57–64 (2014).
Liu, T., Wang, L., Yang, P. & Hu, B. Preparation of nanometer CuFe2O4 by auto-combustion and its catalytic activity on the thermal decomposition of ammonium perchlorate. Mater. Lett. 62(24), 4056–4058 (2008).
Abdellatif, M. H. et al. Effect of Jahn-Teller distortion on the short range magnetic order in copper ferrite. J. Magn. Magn. Mater. 424, 402–409 (2017).
Ansari, F., Sobhani, A. & Salavati-Niasari, M. Sol-gel auto-combustion synthesis of PbFe12O19 using maltose as a novel reductant. RSC Adv. 4, 63946–63950 (2014).
Ansari, F., Sobhani, A. & Salavati-Niasari, M. Facile synthesis, characterization and magnetic property of CuFe12O19 nanostructures via a sol-gel auto-combustion process. J. Magn. Magn. Mater. 401, 362–369 (2016).
Loche, D., Casula, M. F., Falqui, A., Marras, S. & Corrias, A. Preparation of Mn, Ni, Co ferrite highly porous silica nanocomposite aerogels by an urea-assisted sol–gel procedure. J. Nanosci. Nanotechno. 10(2), 1008–1016 (2010).
Casula, M. F., Loche, D., Marras, S., Paschina, G. & Corrias, A. Role of urea in the preparation of highly porous nanocomposite aerogels. Langmuir 23(7), 3509–3512 (2007).
Kucera, M. & Brom, P. Magneto-optical properties of nano-crystalline cubic and tetragonal copper ferrite thin films. J. Appl. Phys. 117, 17B738 (2015).
Balagurov, A. M., Bobrikov, I. A., Maschenko, M. S., Sangaa, D. & Simkin, V. G. Structural phase transition in CuFe2O4 spinel. Crystallogr. Rep. 58(5), 710–717 (2013).
Balagurov, A. M., Bobrikov, I. A., Pomjakushin, V. Y., Sheptyakov, D. V. & Yushankhai, V. Y. Interplay between structural and magnetic phase transitions in copper ferrite studied with high-resolution neutron diffraction. J. Magn. Magn. Mater. 374, 591–599 (2015).
Kester, E. & Gillot, B. Cation distribution, thermodynamic and kinetics considerations in nanoscaled copper ferrite spinels. New experimental approach by XPS and new results both in the bulk and on the grain boundary. J. Phys. Chem. Solids 59(8), 1259–1269 (1998).
Kester, E. et al. Thermal behavior and cation distribution of submicron copper ferrite spinels CuxFe3−xO4 (0 ≤ x ≤ 0.5) studied by DTG, FTIR, and XPS. J. Solid State Chem. 126(1), 7–14 (1996).
Xing, Z., Ju, Z., Yang, J., Xu, H. & Qian, Y. One-step solid state reaction to selectively fabricate cubic and tetragonal CuFe2O4 anode material for high power lithium ion batteries. Electrochim. Acta 102, 51–57 (2013).
Kim, K. J., Lee, J. H. & Lee, S. H. Magneto-optical investigation of spinel ferrite CuFe2O4: observation of Jahn–Teller effect in Cu2+ ion. J. Magn. Magn. Mater. 279(2), 173–177 (2004).
Nedkov, I. & Magnetic structure and collective Jahn–Teller distortions in nanostructured particles of CuFe2O4. Appl. Surf. Sci. 253(5), 2589–2596 (2006).
Bullita, S. et al. ZnFe2O4 nanoparticles dispersed in a highly porous silica aerogel matrix: a magnetic study. Phys. Chem. Chem. Phys. 16, 4843–4852 (2014).
Carta, D. et al. A structural and magnetic investigation of the inversion degree in ferrite nanocrystals MFe2O4 (M = Mn, Co, Ni). J. Phys. Chem. C 113(20), 8606–8615 (2009).
Carta, D., Loche, D., Mountjoy, G., Navarra, G. & Corrias, A. NiFe2O4 nanoparticles dispersed in an aerogel silica matrix: an X-ray absorption study. J. Phys. Chem. C 112(40), 15623–15630 (2008).
Carta, D. et al. X-ray absorption investigation of the formation of cobalt ferrite nanoparticles in an aerogel silica matrix. J. Phys. Chem. C 111(17), 6308–6317 (2007).
Carta, D., Casula, M. F., Mountjoy, G. & Corrias, A. Formation and cation distribution in supported manganese ferrite nanoparticles: an X-ray absorption study. Phys. Chem. Chem. Phys. 10(21), 3108–3117 (2008).
Carta, D. et al. An X-ray absorption spectroscopy study of the inversion degree in zinc ferrite nanocrystals dispersed on a highly porous silica aerogel matrix. J. Chem. Phys. 138, 054702 (2013).
Antonello, A. et al. Synergy of miniemulsion and solvothermal conditions for the low-temperature crystallization of magnetic nanostructured transition-metal ferrites. Chem. Mater. 29, 985–987 (2017).
Krishnan, V., Selvan, R. K., Augustin, C. O., Gedanken, A. & Bertagnolli, H. EXAFS and XANES investigations of CuFe2O4 nanoparticles and CuFe2O4-MO2 (M = Sn, Ce) nanocomposites. J. Phys. Chem. C 111(45), 16724–16733 (2007).
Tasca, J. E., Quincoces, C. E., Lavat, A., Alvarez, A. M. & González, M. G. Preparation and characterization of CuFe2O4 bulk catalysts. Ceram. Int. 37(3), 803–812 (2011).
Klug, P. & Alexander, L. E. X-ray Diffraction Procedures (ed. Wiley), New York (1974).
Ravel, B. & Newville, M. Athena, Artemis, Hephaestus: data analysis for X-ray ab-sorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12(4), 537–541 (2005).
PDF card, reference code: 00-034-0425, Prince, E. & Treuting, R. G. Acta Crystallographica 9 (12), 1025–1028 (1956).
PDF card, reference code: 01-077-0010, Verwey, E. J. W. & Heilman, E. L. J. Chem. Phys. 15(4), 174–180 (1947).
Grunes, L. A. Study of the K edges of 3 d transition metals in pure and oxide form by x-ray-absorption spectroscopy. Phys. Rev. B 27(4), 2111 (1983).
Akeyama, K., Kuroda, H. & Kosugi, N. Cu K-edge XANES and Electronic Structure of Trivalent, Divalent, and Monovalent Cu Oxides. Jpn. J. Appl. Phys. 32, 98–100 (1993).
DuBois, J. L. et al. A Systematic K-edge X-ray Absorption Spectroscopic Study of Cu(III) Sites. J. Am. Chem. Soc. 122, 5775–5787 (2000).
Shimizu, K.-I., Maeshima, H., Yoshida, H., Satsuma, A. & Hattori, T. Ligand field effect on the chemical shift in XANES spectra of Cu(II) compounds. Phys. Chem. Chem. Phys. 3, 862–866 (2001).
PDF card, reference code: 00-005-0661, Asbrink, S. & Norrby, L. Acta Crystallographica B (26), 8–15 (1970).
Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council (EP/K50306X/1), by the Fondazione Banco di Sardegna and by the Regione Autonoma della Sardegna (CUP F72F1600307002). The X-ray absorption spectroscopy experiment was carried out with the support of the Diamond Light Source under rapid access on B18, proposal SP-11086. The authors would like to thank Dr Gavin Mountjoy for helpful discussion and Dr Mark Price for technical assistance in FESEM measurements.
Author information
Authors and Affiliations
Contributions
Work has been done through contributions of all authors. F.C. and A.C. wrote the main manuscript text. F.C. prepared all the figures. F.C. and D.L. synthesized the samples. D.L. performed XRD measurements. M.F.C. acquired TEM images and performed physisorption measurements. A.C. and F.C. performed XAFS data analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Caddeo, F., Loche, D., Casula, M.F. et al. Evidence of a cubic iron sub-lattice in t-CuFe2O4 demonstrated by X-ray Absorption Fine Structure. Sci Rep 8, 797 (2018). https://doi.org/10.1038/s41598-017-19045-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-19045-8
This article is cited by
-
RETRACTED ARTICLE: Biosynthesis of Zn-doped CuFe2O4 nanoparticles and their cytotoxic activity
Scientific Reports (2022)
-
Modification in crystal structure of copper ferrite fiber by annealing and its hyperthermia application
Applied Physics A (2021)
-
Sub-lattice of Jahn-Teller centers in hexaferrite crystal
Scientific Reports (2020)
-
Structural analysis and role of cation distribution on the magnetic properties of single phase Ni-doped copper chromium ferrite
Journal of Materials Science: Materials in Electronics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.