The Structure and Composition of Extracted Pectin and Residual Cell Wall Material from Processing Tomato: The Role of a Stepwise Approach versus High-Pressure Homogenization-Facilitated Acid Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blanching of Tomatoes
2.3. Generation of the Alcohol-Insoluble Residue and Cell Wall Material Fractions
2.3.1. Generation of the Alcohol-Insoluble Residue
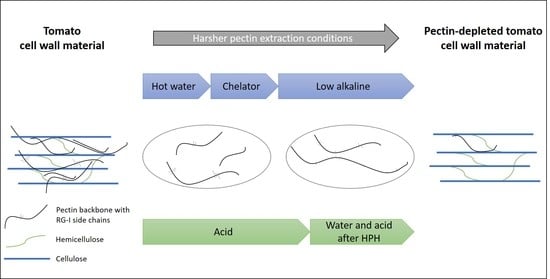
2.3.2. Stepwise Pectin Extraction
Extraction with Hot Water
Extraction with a Chelating Compound
Low-Alkaline Extraction
2.3.3. Acid Extraction and Acid Extraction Facilitated by High-Pressure Homogenization
Acid Extraction
High-Pressure Homogenization of the AcUF
Water and Acid Extraction on the High-Pressure-Homogenized AcUF
2.3.4. Lyophilization of the Extractable and Unextractable Fractions
2.4. Characterization of the Alcohol-Insoluble Residue and Cell Wall Material Fractions
2.4.1. Analysis of the Uronic Acid Content
2.4.2. Analysis of the Neutral Monosaccharide Composition
2.4.3. Analysis of the Degree of Methyl Esterification
2.4.4. Analysis of Molecular Mass Distribution of the Extractable Fractions
2.5. Statistical Analysis
3. Results
3.1. Uronic Acid Extraction Efficiency of the Different Pectin Extraction Procedures
3.2. Chemical Properties of Extractable Fractions in Relation to the Extraction Method
3.2.1. Monosaccharide Composition of the Extractable Fractions
3.2.2. Molecular Mass of the Extractable Fractions
3.2.3. Degree of Methyl Esterification of the WEF, AcEF, AcUF—HPH—WEF and AcUF—HPH—AcEF
3.3. Monosaccharide Composition of the Alcohol-Insoluble Residue and Unextractable Fractions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AcEF | acid-extractable fraction |
| AcUF | acid-unextractable fraction |
| AcUF—HPH—AcEF | acid-extractable fraction of the acid-unextractable fraction subjected to HPH |
| AcUF—HPH—AcUF | acid-unextractable fraction of the acid-unextractable fraction subjected to HPH |
| AcUF—HPH—WEF | water-extractable fraction of the acid-unextractable fraction subjected to HPH |
| AIR | alcohol-insoluble residue |
| Ara | arabinose |
| CDTA | cyclohexane-trans-1,2-diaminetetraacetic acid |
| CEF | chelator-extractable fraction |
| CUF | chelator-unextractable fraction |
| CWM | cell wall material |
| DM | methyl esterification |
| EDTA | ethylenediaminetetraacetic acid |
| EF | extractable fraction |
| FT-IR | Fourier-transform infrared |
| Fuc | fucose |
| Gal | galactose |
| GalA | galacturonic acid |
| Glc | glucose |
| GlcA | glucuronic acid |
| HG | homogalacturonan |
| HPAEC–PAD | high-performance anion exchange chromatography with pulsed amperometric detection |
| HPH | high-pressure homogenization |
| lAEF | low-alkaline-extractable fraction |
| lAUF | low-alkaline-unextractable fraction |
| Man | mannose |
| MM | molecular mass |
| RG-I | rhamnogalacturonan I |
| RG-II | rhamnogalacturonan II |
| Rha | rhamnose |
| UA | uronic acid |
| UF | unextractable fraction |
| WEF | water-extractable fraction |
| Xyl | xylose |
References
- Doblin, M.S.; Pettolino, F.; Bacic, A. Plant cell walls: The skeleton of the plant world. Funct. Plant Biol. 2010, 37, 357. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Jarvis, M.C. Plant cell walls: Supramolecular assemblies. Food Hydrocoll. 2011, 25, 257–262. [Google Scholar] [CrossRef]
- Gomez, L.D.; Steele-King, C.G.; McQueen-Mason, S.J. Sustainable liquid biofuels from biomass: The writing’s on the walls. New Phytol. 2008, 178, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Waldron, K.W.; Parker, M.L.; Smith, A.C. Plant Cell Walls and Food Quality. Compr. Rev. Food Sci. Food Saf. 2003, 2, 128–146. [Google Scholar] [CrossRef]
- Fry, S.C. The structure and functions of xyloglucan. J. Exp. Bot. 1989, 40, 1–11. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef] [PubMed]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T. Xyloglucans in the Primary Cell Wall. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 139–168. [Google Scholar] [CrossRef]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Wall Structure and Wall Loosening. A Look Backwards and Forwards. Plant Physiol. 2001, 125, 131–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popper, Z.A.; Fry, S.C. Widespread Occurrence of a Covalent Linkage between Xyloglucan and Acidic Polysaccharides in Suspension-cultured Angiosperm Cells. Ann. Bot. 2005, 96, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Keegstra, K.; Talmadge, K.W.; Bauer, W.D.; Albersheim, P. The Structure of Plant Cell Walls III. A Model of the Walls of Suspension-Cultured Sycamore Cells Based on the Interconnections of the Macromolecular Components. Plant Physiol. 1973, 51, 188–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zykwinska, A.; Thibault, J.-F.; Ralet, M.-C. Organization of pectic arabinan and galactan side chains in association with cellulose microfibrils in primary cell walls and related models envisaged. J. Exp. Bot. 2007, 58, 1795–1802. [Google Scholar] [CrossRef] [Green Version]
- Zykwinska, A.W.; Ralet, M.-C.J.; Garnier, C.D.; Thibault, J.-F.J. Evidence for In Vitro Binding of Pectin Side Chains to Cellulose. Plant Physiol. 2005, 139, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, D.J. Re-constructing our models of cellulose and primary cell wall assembly. Curr. Opin. Plant Biol. 2014, 22, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.B.; Cosgrove, D.J. A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiol. 2012, 158, 1933–1943. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Wang, X.; Chen, Y.; Wagner, E.; Cosgrove, D.J. Xyloglucan in the primary cell wall: Assessment by FESEM, selective enzyme digestions and nanogold affinity tags. Plant J. 2018, 93, 211–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M.A. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Ciriminna, R.; Chavarría-Hernández, N.; Rodríguez Hernández, A.I.; Pagliaro, M. Pectin: A new perspective from the biorefinery standpoint. Biofuels Bioprod. Biorefin. 2015, 9, 368–377. [Google Scholar] [CrossRef]
- Yapo, B.M.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem. 2007, 100, 1356–1364. [Google Scholar] [CrossRef]
- Levigne, S.; Ralet, M.-C.; Thibault, J.-F. Characterisation of pectins extracted from fresh sugar beet under different conditions using an experimental design. Carbohydr. Polym. 2002, 49, 145–153. [Google Scholar] [CrossRef]
- Koubala, B.B.; Kansci, G.; Mbome, L.I.; Crépeau, M.-J.; Thibault, J.-F.; Ralet, M.-C. Effect of extraction conditions on some physicochemical characteristics of pectins from “Améliorée” and “Mango” mango peels. Food Hydrocoll. 2008, 22, 1345–1351. [Google Scholar] [CrossRef]
- Thibault, J.-F.; Renard, C.M.G.C.; Axelos, M.A.V.; Roger, P.; Crépeau, M.-J. Studies of the length of homogalacturonic regions in pectins by acid hydrolysis. Carbohydr. Res. 1993, 238, 271–286. [Google Scholar] [CrossRef]
- Fry, S.C. Cross-Linking of Matrix Polymers in the Growing Cell Walls of Angiosperms. Annu. Rev. Plant Physiol. 1986, 37, 165–186. [Google Scholar] [CrossRef]
- Christiaens, S.; Van Buggenhout, S.; Houben, K.; Fraeye, I.; Van Loey, A.M.; Hendrickx, M.E. Towards a better understanding of the pectin structure–function relationship in broccoli during processing: Part I—Macroscopic and molecular analyses. Food Res. Int. 2011, 44, 1604–1612. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Thibault, J.-F. Structure and properties of apple and sugar-beet pectins extracted by chelating agents. Carbohydr. Res. 1993, 244, 99–114. [Google Scholar] [CrossRef]
- Basanta, M.F.; Ponce, N.M.A.; Rojas, A.M.; Stortz, C.A. Effect of extraction time and temperature on the characteristics of loosely bound pectins from Japanese plum. Carbohydr. Polym. 2012, 89, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Houben, K.; Jolie, R.P.; Fraeye, I.; Van Loey, A.M.; Hendrickx, M.E. Comparative study of the cell wall composition of broccoli, carrot, and tomato: Structural characterization of the extractable pectins and hemicelluloses. Carbohydr. Res. 2011, 346, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, S.; Uwibambe, D.; Uyttebroek, M.; Van Droogenbroeck, B.; Van Loey, A.M.; Hendrickx, M.E. Pectin characterisation in vegetable waste streams: A starting point for waste valorisation in the food industry. LWT Food Sci. Technol. 2015, 61, 275–282. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Voragen, A.G.J.; Thibault, J.F.; Pilnik, W. Studies on Apple Protopectin: I. Extraction of Insoluble Pectin by Chemical Means. Carbohydr. Polym. 1990, 12, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Broxterman, S.E.; Schols, H.A. Interactions between pectin and cellulose in primary plant cell walls. Carbohydr. Polym. 2018, 192, 263–272. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Ginies, C. Comparison of the cell wall composition for flesh and skin from five different plums. Food Chem. 2009, 114, 1042–1049. [Google Scholar] [CrossRef]
- Seymour, G.B.; Colquhoun, I.J.; DuPont, M.S.; Parsley, K.R.; Selvendran, R.R. Composition and structural features of cell wall polysaccharides from tomato fruits. Phytochemistry 1990, 29, 725–731. [Google Scholar] [CrossRef]
- Van Buggenhout, S.; Wallecan, J.; Christiaens, S.; Debon, S.J.J.; Desmet, C.; Van Loey, A.; Hendrickx, M.; Mazoyer, J. Influence of high-pressure homogenization on functional properties of orange pulp. Innov. Food Sci. Emerg. Technol. 2015, 30, 51–60. [Google Scholar] [CrossRef]
- Christiaens, S.; Van Buggenhout, S.; Chaula, D.; Moelants, K.; David, C.C.; Hofkens, J.; Van Loey, A.M.; Hendrickx, M.E. In situ pectin engineering as a tool to tailor the consistency and syneresis of carrot purée. Food Chem. 2012, 133, 146–155. [Google Scholar] [CrossRef]
- Christiaens, S.; Mbong, V.B.; Van Buggenhout, S.; David, C.C.; Hofkens, J.; Van Loey, A.M.; Hendrickx, M.E. Influence of processing on the pectin structure–function relationship in broccoli purée. Innov. Food Sci. Emerg. Technol. 2012, 15, 57–65. [Google Scholar] [CrossRef]
- Christiaens, S.; Van Buggenhout, S.; Houben, K.; Chaula, D.; Van Loey, A.M.; Hendrickx, M.E. Unravelling process-induced pectin changes in the tomato cell wall: An integrated approach. Food Chem. 2012, 132, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Broxterman, S.E.; Schols, H.A. Characterisation of pectin-xylan complexes in tomato primary plant cell walls. Carbohydr. Polym. 2018, 197, 269–276. [Google Scholar] [CrossRef]
- Raiola, A.; Rigano, M.M.; Calafiore, R.; Frusciante, L.; Barone, A. Enhancing the Health-Promoting Effects of Tomato Fruit for Biofortified Food. Mediat. Inflamm. 2014, 2014, 139873. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Moon, S.H.; Keum, Y.-S. An updated review on use of tomato pomace and crustacean processing waste to recover commercially vital carotenoids. Food Res. Int. 2018, 108, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Neckebroeck, B.; Verkempinck, S.H.E.; Van Audenhove, J.; Bernaerts, T.; de Wilde d’Estmael, H.; Hendrickx, M.E.; Van Loey, A.M. Structural and emulsion stabilizing properties of pectin rich extracts obtained from different botanical sources. Food Res. Int. 2021, 141, 110087. [Google Scholar] [CrossRef] [PubMed]
- McFeeters, R.F.; Armstrong, S.A. Measurement of pectin methylation in plant cell walls. Anal. Biochem. 1984, 139, 212–217. [Google Scholar] [CrossRef]
- Willemsen, K.L.D.D.; Panozzo, A.; Moelants, K.; Debon, S.J.J.; Desmet, C.; Cardinaels, R.; Moldenaers, P.; Wallecan, J.; Hendrickx, M.E.G. Physico-chemical and viscoelastic properties of high pressure homogenized lemon peel fiber fraction suspensions obtained after sequential pectin extraction. Food Hydrocoll. 2017, 72, 358–371. [Google Scholar] [CrossRef] [Green Version]
- Bernaerts, T.M.M.; Gheysen, L.; Kyomugasho, C.; Kermani, Z.J.; Vandionant, S.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Comparison of microalgal biomasses as functional food ingredients: Focus on the composition of cell wall related polysaccharides. Algal Res. 2018, 32, 150–161. [Google Scholar] [CrossRef]
- Ahmed, A.E.R.; Labavitch, J.M. A simplified method for accurate determination of cell wall uronide content. J. Food Biochem. 1977, 1, 361–365. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method of quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Yeats, T.; Vellosillo, T.; Sorek, N.; Ibáñez, A.; Bauer, S. Rapid Determination of Cellulose, Neutral Sugars, and Uronic Acids from Plant Cell Walls by One-Step Two-Step Hydrolysis and HPAEC-PAD. Bio-Protocol 2016, 6, e1978. [Google Scholar] [CrossRef] [Green Version]
- Neckebroeck, B.; Verkempinck, S.H.E.; Vaes, G.; Wouters, K.; Magnée, J.; Hendrickx, M.E.; Van Loey, A.M. Advanced insight into the emulsifying and emulsion stabilizing capacity of carrot pectin subdomains. Food Hydrocoll. 2020, 102, 105594. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.M.; Hendrickx, M.E. FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit- and vegetable-based matrices. Food Chem. 2015, 176, 82–90. [Google Scholar] [CrossRef]
- Kaya, M.; Sousa, A.G.; Crépeau, M.-J.; Sørensen, S.O.; Ralet, M.-C. Characterization of citrus pectin samples extracted under different conditions: Influence of acid type and pH of extraction. Ann. Bot. 2014, 114, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Shpigelman, A.; Kyomugasho, C.; Christiaens, S.; Van Loey, A.M.; Hendrickx, M.E. Thermal and high pressure high temperature processes result in distinctly different pectin non-enzymatic conversions. Food Hydrocoll. 2014, 39, 251–263. [Google Scholar] [CrossRef]
- Reinders, G.; Thier, H.-P. Non-starch polysaccharides of tomatoes I. Characterizing pectins and hemicelluloses. Eur. Food Res. Technol. 1999, 209, 43–46. [Google Scholar] [CrossRef]
- Denman, L.J.; Morris, G.A. An experimental design approach to the chemical characterisation of pectin polysaccharides extracted from Cucumis melo Inodorus. Carbohydr. Polym. 2015, 117, 364–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moelants, K.R.N.; Jolie, R.P.; Palmers, S.K.J.; Cardinaels, R.; Christiaens, S.; Van Buggenhout, S.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. The Effects of Process-Induced Pectin Changes on the Viscosity of Carrot and Tomato Sera. Food Bioprocess Technol. 2013, 6, 2870–2883. [Google Scholar] [CrossRef]
- Redgwell, R.J.; MacRae, E.; Hallett, I.; Fischer, M.; Perry, J.; Harker, R. In vivo and in vitro swelling of cell walls during fruit ripening. Planta 1997, 203, 162–173. [Google Scholar] [CrossRef]
- Sankaran, A.K.; Nijsse, J.; Bialek, L.; Bouwens, L.; Hendrickx, M.E.; Van Loey, A.M. Effect of enzyme homogenization on the physical properties of carrot cell wall suspensions. Food Bioprocess Technol. 2015, 8, 1377–1385. [Google Scholar] [CrossRef]
- Guillon, F.; Philippe, S.; Bouchet, B.; Devaux, M.F.; Frasse, P.; Jones, B.; Bouzayen, M.; Lahaye, M. Down-regulation of an Auxin Response Factor in the tomato induces modification of fine pectin structure and tissue architecture. J. Exp. Bot. 2008, 59, 273–288. [Google Scholar] [CrossRef] [Green Version]
- Schultink, A.; Liu, L.; Zhu, L.; Pauly, M. Structural Diversity and Function of Xyloglucan Sidechain Substituents. Plants 2014, 3, 526–542. [Google Scholar] [CrossRef] [Green Version]
- Ordaz-Ortiz, J.J.; Marcus, S.E.; Knox, J.P. Cell Wall Microstructure Analysis Implicates Hemicellulose Polysaccharides in Cell Adhesion in Tomato Fruit Pericarp Parenchyma. Mol. Plant 2009, 2, 910–921. [Google Scholar] [CrossRef]
- Fraeye, I.; Duvetter, T.; Doungla, E.; Van Loey, A.; Hendrickx, M. Fine-tuning the properties of pectin–calcium gels by control of pectin fine structure, gel composition and environmental conditions. Trends Food Sci. Technol. 2010, 21, 219–228. [Google Scholar] [CrossRef]
- Pagán, J.; Ibarz, A. Extraction and rheological properties of pectin from fresh peach pomace. J. Food Eng. 1999, 39, 193–201. [Google Scholar] [CrossRef]
- Lin, H.; Aizawa, K.; Inakuma, T.; Yamauchi, R.; Kato, K. Physical properties of water-soluble pectins in hot- and cold-break tomato pastes. Food Chem. 2005, 93, 403–408. [Google Scholar] [CrossRef]
- Shpigelman, A.; Kyomugasho, C.; Christiaens, S.; Van Loey, A.M.; Hendrickx, M.E. The effect of high pressure homogenization on pectin: Importance of pectin source and pH. Food Hydrocoll. 2015, 43, 189–198. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Munyensanga, C.; Celus, M.; Van de Walle, D.; Dewettinck, K.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Molar mass influence on pectin-Ca2+ adsorption capacity, interaction energy and associated functionality: Gel microstructure and stiffness. Food Hydrocoll. 2018, 85, 331–342. [Google Scholar] [CrossRef]
- Fracasso, A.F.; Perussello, C.A.; Carpiné, D.; de Oliveira Petkowicz, C.L.; Haminiuk, C.W.I. Chemical modification of citrus pectin: Structural, physical and rheologial implications. Int. J. Biol. Macromol. 2018, 109, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Sila, D.N.; Smout, C.; Elliot, F.; Van Loey, A.; Hendrickx, M. Non-enzymatic Depolymerization of Carrot Pectin: Toward a Better Understanding of Carrot Texture during Thermal Processing. J. Food Sci. 2006, 71, E1–E9. [Google Scholar] [CrossRef]
- Hyodo, H.; Terao, A.; Furukawa, J.; Sakamoto, N.; Yurimoto, H.; Satoh, S.; Iwai, H. Tissue Specific Localization of Pectin–Ca2+ Cross-Linkages and Pectin Methyl-Esterification during Fruit Ripening in Tomato (Solanum lycopersicum). PLoS ONE 2013, 8, e78949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraeye, I.; De Roeck, A.; Duvetter, T.; Verlent, I.; Hendrickx, M.; Van Loey, A. Influence of pectin properties and processing conditions on thermal pectin degradation. Food Chem. 2007, 105, 555–563. [Google Scholar] [CrossRef]
- Zykwinska, A.; Gaillard, C.; Buléon, A.; Pontoire, B.; Garnier, C.; Thibault, J.-F.; Ralet, M.-C. Assessment of In Vitro Binding of Isolated Pectic Domains to Cellulose by Adsorption Isotherms, Electron Microscopy, and X-ray Diffraction Methods. Biomacromolecules 2007, 8, 223–232. [Google Scholar] [CrossRef]
- Brett, C.T.; Baydoun, E.A.-H.; Abdel-Massih, R.M. Pectin—Xyloglucan linkages in type I primary cell walls of plants. Plant Biosyst. 2005, 139, 54–59. [Google Scholar] [CrossRef]
- Gu, J.; Catchmark, J.M. The impact of cellulose structure on binding interactions with hemicellulose and pectin. Cellulose 2013, 20, 1613–1627. [Google Scholar] [CrossRef]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging concepts in the nutraceutical and functional properties of pectin—A Review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef] [PubMed]



| EF | Monosaccharide Content (mg/g) | DM (%) | MM (kDa) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rha | Ara | Gal | Glc | Xyl | Man | UA | |||
| WEF | 9.8 ± 0.9 | 18.6 ± 1.6 | 23.7 ± 1.0 | 15.8 ± 0.9 | 11.4 ± 0.8 | 8.4 ± 0.2 | 573.9 ± 8.1 | 73.8 ± 2.7 | 114.8 ± 3.1 |
| CEF | 3.5 ± 0.4 | 5.1 ± 0.4 | 7.2 ± 0.6 | 4.7 ± 0.8 | 0.8 ± 0.4 | 1.8 ± 0.3 | 203.0 ± 14.2 | n.d. | 233.0 ± 4.8 |
| lAEF | 15.2 ± 3.0 | 9.8 ± 1.2 | 14.5 ± 1.2 | 0.9 ± 0.2 | 1.9 ± 0.5 | <d.l. | 400.2 ± 21.9 | n.d. | 451.0 ± 42.5 |
| AcEF | 16.4 ± 1.2 | 15.5 ± 0.2 | 19.6 ± 0.5 | 8.9 ± 0.2 | 2.1 ± 0.2 | 1.2 ± 0.3 | 558.5 ± 31.6 | 66.0 ± 3.6 | 270.3 ± 10.1 |
| AcUF—HPH—WEF | 29.3 ± 2.0 | 8.2 ± 0.5 | 19.3 ± 1.2 | 2.8 ± 0.5 | 7.3 ± 0.5 | <d.l. | 680.5 ± 49.6 | 60.5 ± 0.9 | 1507.5 ± 85.4 |
| AcUF—HPH—AcEF | 24.0 ± 0.9 | 5.4 ± 1.1 | 16.5 ± 1.4 | 4.6 ± 0.7 | 4.5 ± 0.2 | 0.9 ± 0.7 | 560.8 ± 46.5 | 47.0 ± 2.1 | 509.5 ± 52.8 |
| EF | Contribution of HG (mol%) | Contribution of RG-I (mol%) | Contribution of RG-I to the Pectin Backbone (-) | Branching of RG-I (-) | Purity of the Pectin Extract (-) |
|---|---|---|---|---|---|
| UA–Rha | 2Rha + Ara + Gal | Rha/UA | (Ara + Gal)/Rha | (Rha + Ara + Gal + UA)/(Glc + Man) | |
| WEF | 83.2 ± 1.6 | 10.8 ± 0.5 | 0.020 ± 0.002 | 4.3 ± 0.5 | 24 ± 1 |
| CEF | 86.6 ± 8.3 | 9.9 ± 0.6 | 0.021 ± 0.003 | 3.4 ± 0.4 | 32 ± 4 |
| lAEF | 84.9 ± 6.6 | 14.3 ± 1.7 | 0.045 ± 0.009 | 1.6 ± 0.3 | 387 ± 90 |
| AcEF | 85.2 ± 6.7 | 12.6 ± 0.6 | 0.035 ± 0.003 | 2.1 ± 0.2 | 57 ± 4 |
| AcUF—HPH—WEF | 85.1 ± 8.8 | 13.3 ± 0.9 | 0.051 ± 0.005 | 0.9 ± 0.1 | 244 ± 45 |
| AcUF—HPH—AcEF | 85.1 ± 10.0 | 13.0 ± 0.9 | 0.051 ± 0.005 | 0.9 ± 0.1 | 105 ± 20 |
| AIR or UF | Monosaccharide Content (mg/g) | ||||||
|---|---|---|---|---|---|---|---|
| Rha | Ara | Gal | Glc | Xyl | Man | UA | |
| AIR | 6.3 ± 0.1 | 12.4 ± 1.1 | 14.1 ± 1.0 | 293.1 ± 13.8 | 34.9 ± 2.2 | 26.4 ± 1.8 | 262.1 ± 12.9 |
| CUF | 6.6 ± 0.5 | 12.9 ± 1.1 | 16.9 ± 1.3 | 406.9 ± 31.3 | 52.2 ± 5.1 | 39.8 ± 2.2 | 163.4 ± 11.6 |
| lAUF | 2.3 ± 0.4 | 12.4 ± 1.3 | 16.3 ± 1.9 | 471.6 ± 17.5 | 58.7 ± 1.7 | 44.7 ± 1.3 | 40.2 ± 2.8 |
| AcUF | 3.9 ± 0.6 | 6.0 ± 1.0 | 14.0 ± 1.1 | 441.5 ± 24.5 | 55.6 ± 2.6 | 43.2 ± 2.3 | 182.9 ± 6.3 |
| AcUF—HPH—AcUF | 1.6 ± 0.3 | 2.8 ± 0.7 | 14.3 ± 1.8 | 509.6 ± 31.1 | 63.9 ± 4.3 | 52.5 ± 2.5 | 79.4 ± 2.2 |
| AIR or UF | Contribution of the Pectin Backbone (mol%) | Contribution of Mannans to Hemicellulose (-) | Ratio of Typical Hemicellulose Monosaccharides to Glucose (-) | Contribution of Hemicellulose and Cellulose (mol%) |
|---|---|---|---|---|
| UA + Rha | Man/Xyl | (Xyl + Man)/Glc | Glc + Xyl + Man | |
| AIR | 39.0 ± 2.2 | 0.63 ± 0.06 | 0.23 ± 0.02 | 56.4 ± 2.6 |
| CUF | 22.7 ± 1.9 | 0.64 ± 0.07 | 0.25 ± 0.03 | 72.7 ± 5.4 |
| lAUF | 6.1 ± 0.4 | 0.63 ± 0.03 | 0.24 ± 0.01 | 89.2 ± 3.3 |
| AcUF | 23.3 ± 1.1 | 0.65 ± 0.05 | 0.25 ± 0.02 | 73.9 ± 3.9 |
| AcUF—HPH—AcUF | 10.3 ± 0.5 | 0.69 ± 0.06 | 0.25 ± 0.02 | 87.3 ± 5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Audenhove, J.; Bernaerts, T.; De Smet, V.; Delbaere, S.; Van Loey, A.M.; Hendrickx, M.E. The Structure and Composition of Extracted Pectin and Residual Cell Wall Material from Processing Tomato: The Role of a Stepwise Approach versus High-Pressure Homogenization-Facilitated Acid Extraction. Foods 2021, 10, 1064. https://doi.org/10.3390/foods10051064
Van Audenhove J, Bernaerts T, De Smet V, Delbaere S, Van Loey AM, Hendrickx ME. The Structure and Composition of Extracted Pectin and Residual Cell Wall Material from Processing Tomato: The Role of a Stepwise Approach versus High-Pressure Homogenization-Facilitated Acid Extraction. Foods. 2021; 10(5):1064. https://doi.org/10.3390/foods10051064
Chicago/Turabian StyleVan Audenhove, Jelle, Tom Bernaerts, Victor De Smet, Sophie Delbaere, Ann M. Van Loey, and Marc E. Hendrickx. 2021. "The Structure and Composition of Extracted Pectin and Residual Cell Wall Material from Processing Tomato: The Role of a Stepwise Approach versus High-Pressure Homogenization-Facilitated Acid Extraction" Foods 10, no. 5: 1064. https://doi.org/10.3390/foods10051064