The Effect of Different Compositions and Concentrations of Etidronate-Containing Irrigants on the Antibacterial Activity of Sodium Hypochlorite against Enterococcus faecalis and Candida albicans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Irrigant Preparation, and pH and Temperature Measurement
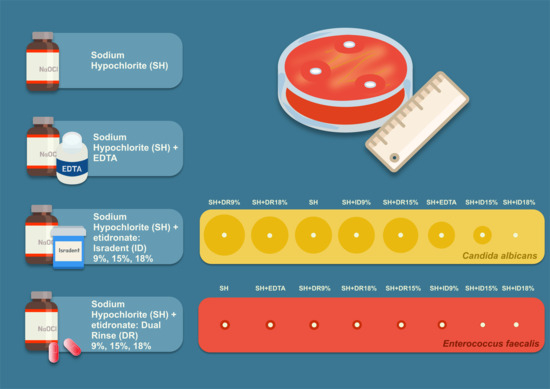
- SH—sodium hypochlorite (“Hypochloran-3”, Omegadent, Moscow, Russia);
- SH + EDTA—sodium hypochlorite (“Hypochloran-3”, Omegadent, Moscow, Russia) mixed with 17% EDTA (“MD-Cleanser”, Metabiomed, Cheongju, Republic of Korea);
- SH + DR—sodium hypochlorite (“Hypochloran-3”, Omegadent, Moscow, Russia) mixed with Dual Rinse® HEDP (Medcem, Weinfelden, Switzerland);
- SH + ID—sodium hypochlorite (“Hypochloran-3”, Omegadent, Moscow, Russia mixed with Isradent® HEDP (“HEBP Etidronic acid”, Isradent, Tyumen, Russia);
- DW—distilled water (control).
2.2. Antiseptic Effect Assessment
2.2.1. Candida albicans
Identification of a Pure Culture
Antiseptic Susceptibility Testing
2.2.2. Enterococcus faecalis
Identification of a Pure Culture
Antiseptic Susceptibility Testing
2.3. Statistical Analyses
3. Results
3.1. pH and Temperature Measurements of the Tested Solutions
3.2. Antiseptic Effect Assessment
3.2.1. Antiseptic Effect of the Tested Solutions against C. albicans
3.2.2. Antiseptic Effect of the Tested Solutions against E. faecalis
3.3. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torabinejad, M.; Fouad, A.F.; Shabahang, S. Endodontics Principles and Practice, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780323624367. [Google Scholar]
- Violich, D.R.; Chandler, N.P. The Smear Layer in Endodontics: A Review. Int. Endod. J. 2010, 43, 2–15. [Google Scholar] [CrossRef]
- Aksel, H.; Küçükkaya Eren, S.; Puralı, N.; Serper, A.; Azim, A.A. Efficacy of Different Irrigant Protocols and Application Systems on Sealer Penetration Using a Stepwise CLSM Analysis. Microsc. Res. Tech. 2017, 80, 1323–1327. [Google Scholar] [CrossRef]
- Matos, F.D.S.; da Silva, F.R.; Paranhos, L.R.; Moura, C.C.G.; Bresciani, E.; Valera, M.C. The Effect of 17% EDTA and QMiX Ultrasonic Activation on Smear Layer Removal and Sealer Penetration: Ex Vivo Study. Sci. Rep. 2020, 10, 10311. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, P.; Varughese, A.A.; Sharma, S.; Subbarao, C.V.; Zehnder, M.; De-Deus, G. Continuous Chelation Irrigation Improves the Adhesion of Epoxy Resin-Based Root Canal Sealer to Root Dentine. Int. Endod. J. 2012, 45, 1097–1102. [Google Scholar] [CrossRef]
- Shahravan, A.; Haghdoost, A.A.; Adl, A.; Rahimi, H.; Shadifar, F. Effect of Smear Layer on Sealing Ability of Canal Obturation: A Systematic Review and Meta-Analysis. J. Endod. 2007, 33, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Drake, D.R.; Wiemann, A.H.; Rivera, E.M.; Walton, R.E. Bacterial Retention in Canal Walls in Vitro: Effect of Smear Layer. J. Endod. 1994, 20, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Basrani, B.; Haapasalo, M. Update on Endodontic Irrigating Solutions. Endod. Top. 2012, 27, 74–102. [Google Scholar] [CrossRef]
- Hülsmann, M.; Heckendorff, M.; Lennon, Á. Chelating Agents in Root Canal Treatment: Mode of Action and Indications for Their Use. Int. Endod. J. 2003, 36, 810–830. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Shalavi, S.; Yaripour, S.; Kinoshita, J.-I.; Manabe, A.; Kobayashi, M.; Giardino, L.; Palazzi, F.; Sharifi, F.; Jafarzadeh, H. Smear Layer Removing Ability of Root Canal Irrigation Solutions: A Review. J. Contemp. Dent. Pract. 2019, 20, 395–402. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Arias-Moliz, M.T. Present Status and Future Directions—Irrigants and Irrigation Methods. Int. Endod. J. 2022, 55, 588–612. [Google Scholar] [CrossRef]
- Machado, R.; Garcia, L.D.F.R.; da Silva Neto, U.X.; da Cruz Filho, A.D.M.; Silva, R.G.; Vansan, L.P. Evaluation of 17% EDTA and 10% Citric Acid in Smear Layer Removal and Tubular Dentin Sealer Penetration. Microsc. Res. Tech. 2018, 81, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Alakshar, A.; Saleh, A.R.M.; Gorduysus, M.O. Debris and Smear Layer Removal from Oval Root Canals Comparing XP-Endo Finisher, EndoActivator, and Manual Irrigation: A SEM Evaluation. Eur. J. Dent. 2020, 14, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidi, E.; Psimma, Z.; Chávez de Paz, L.E.; Boutsioukis, C. Apical Negative Pressure Irrigation versus Syringe Irrigation: A Systematic Review of Cleaning and Disinfection of the Root Canal System. Int. Endod. J. 2017, 50, 1034–1054. [Google Scholar] [CrossRef] [PubMed]
- Badami, V.; Akarapu, S.; Kethineni, H.; Mittapalli, S.P.; Bala, K.R.; Fatima, S.F. Efficacy of Laser-Activated Irrigation Versus Ultrasonic-Activated Irrigation: A Systematic Review. Cureus 2023, 15, e36352. [Google Scholar] [CrossRef] [PubMed]
- Solakoğlu, E.; Topçuoğlu, H.S.; Düzgün, S. Effect of Different Final Irrigation Agitation Techniques on Root Canal Dentin Tubule Penetration of Nanoparticle Calcium Hydroxide Dressing. Aust. Endod. J. 2023, 49, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Mikheikina, A.; Novozhilova, N.; Polyakova, M.; Sokhova, I.; Mun, A.; Zaytsev, A.; Babina, K.; Makeeva, I. Knowledge, Attitude, and Practice towards Chelating Agents in Endodontic Treatment among Dental Practitioners. Dent. J. 2023, 11, 156. [Google Scholar] [CrossRef]
- Tsotsis, P.; Dunlap, C.; Scott, R.; Arias, A.; Peters, O.A. A Survey of Current Trends in Root Canal Treatment: Access Cavity Design and Cleaning and Shaping Practices. Aust. Endod. J. 2021, 47, 27–33. [Google Scholar] [CrossRef]
- Gopikrishna, V.; Pare, S.; Pradeep Kumar, A.; Lakshmi Narayanan, L. Irrigation Protocol among Endodontic Faculty and Post-Graduate Students in Dental Colleges of India: A Survey. J. Conserv. Dent. 2013, 16, 394–398. [Google Scholar] [CrossRef]
- Alzamzami, Z.T.; Alqurashi, A.A.; Almansour, L.A.; Ashi, H.M.; Abulhamael, A.M.; Alghamdi, F.T.; Albahiti, M.T. Current Trends in Irrigation Solution and Adjunct Use During Endodontic Therapy among Dental Professionals in Jeddah, Saudi Arabia: A Cross-Sectional Study. Cureus 2022, 14, e32168. [Google Scholar] [CrossRef]
- Natanasabapathy, V.; Durvasulu, A.; Krithikadatta, J.; Namasivayam, A.; Deivanayagam, K.; Manali, S.; Sureshbabu, N.M. Current Trends in the Use of Irrigant Activation Techniques among Endodontists & Post-Graduate Dental Students in India—A Knowledge, Attitude and Practice Based Survey. Eur. Endod. J. 2020, 5, 73–80. [Google Scholar] [CrossRef]
- Gawdat, S.I.; Bedier, M.M. Influence of Dual Rinse Irrigation on Dentinal Penetration of a Bioceramic Root Canal Sealer: A Conofocal Microscopic Analysis. Aust. Endod. J. 2022, 48, 481–486. [Google Scholar] [CrossRef]
- Zehnder, M.; Schmidlin, P.; Sener, B.; Waltimo, T. Chelation in Root Canal Therapy Reconsidered. J. Endod. 2005, 31, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Soligo, L.T.; Lodi, E.; Farina, A.P.; Souza, M.A.; Vidal, C.D.M.P.; Cecchin, D. Antibacterial Efficacy of Synthetic and Natural-Derived Novel Endodontic Irrigant Solutions. Braz. Dent. J. 2018, 29, 459–464. [Google Scholar] [CrossRef]
- Wagner, M.H.; da Rosa, R.A.; de Figueiredo, J.A.P.; Duarte, M.A.H.; Pereira, J.R.; Só, M.V.R. Final Irrigation Protocols May Affect Intraradicular Dentin Ultrastructure. Clin. Oral Investig. 2017, 21, 2173–2182. [Google Scholar] [CrossRef]
- Viola, K.S.; Coaguila-Llerena, H.; Rodrigues, E.M.; Santos, C.S.; Chávez-Andrade, G.M.; Magro, M.G.; Tanomaru-Filho, M.; Guerreiro-Tanomaru, J.M.; Faria, G. Different Formulations of Peracetic Acid: Effects on Smear Layer Removal, Dentine Erosion, Cytotoxicity and Antibiofilm Activity. J. Appl. Oral Sci. 2022, 30, e20210575. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, D.P.D.C.; Farina, A.P.; Barcellos, R.; Souza, M.A.; Borba, M.; Bedran-Russo, A.K.; Bello, Y.D.; Pimenta Vidal, C.D.M.; Cecchin, D. Effect of a New Irrigant Solution Containing Glycolic Acid on Smear Layer Removal and Chemical/Mechanical Properties of Dentin. Sci. Rep. 2020, 10, 7313. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Pondrelli, A.; Zamparini, F.; Prati, C.; Spagnuolo, G. Demineralization, Collagen Modification and Remineralization Degree of Human Dentin after EDTA and Citric Acid Treatments. Materials 2018, 12, 25. [Google Scholar] [CrossRef]
- Ballal, N.V.; Jain, I.; Tay, F.R. Evaluation of the Smear Layer Removal and Decalcification Effect of QMix, Maleic Acid and EDTA on Root Canal Dentine. J. Dent. 2016, 51, 62–68. [Google Scholar] [CrossRef]
- Solana, C.; Ruiz-Linares, M.; Baca, P.; Valderrama, M.J.; Arias-Moliz, M.T.; Ferrer-Luque, C.M. Antibiofilm Activity of Sodium Hypochlorite and Alkaline Tetrasodium EDTA Solutions. J. Endod. 2017, 43, 2093–2096. [Google Scholar] [CrossRef]
- Wright, P.P.; Cooper, C.; Kahler, B.; Walsh, L.J. From an Assessment of Multiple Chelators, Clodronate Has Potential for Use in Continuous Chelation. Int. Endod. J. 2020, 53, 122–134. [Google Scholar] [CrossRef]
- Biel, P.; Mohn, D.; Attin, T.; Zehnder, M. Interactions between the Tetrasodium Salts of EDTA and 1-Hydroxyethane 1,1-Diphosphonic Acid with Sodium Hypochlorite Irrigants. J. Endod. 2017, 43, 657–661. [Google Scholar] [CrossRef]
- Ali, A.; Bhosale, A.; Pawar, S.; Kakti, A.; Bichpuriya, A.; Agwan, M.A. Current Trends in Root Canal Irrigation. Cureus 2022, 14, e24833. [Google Scholar] [CrossRef]
- Borges, M.M.B.; Dijkstra, R.J.B.; de Andrade, F.B.; Duarte, M.A.H.; Versluis, M.; van der Sluis, L.W.M.; Petridis, X. The Response of Dual-species Bacterial Biofilm to 2% and 5% NaOCl Mixed with Etidronic Acid: A Laboratory Real-time Evaluation Using Optical Coherence Tomography. Int. Endod. J. 2022, 55, 758. [Google Scholar] [CrossRef]
- Campello, A.F.; Rodrigues, R.C.V.; Alves, F.R.F.; Miranda, K.R.; Brum, S.C.; Mdala, I.; Siqueira, J.F.; Rôças, I.N. Enhancing the Intracanal Antibacterial Effects of Sodium Hypochlorite with Etidronic Acid or Citric Acid. J. Endod. 2022, 48, 1161–1168. [Google Scholar] [CrossRef]
- De-Deus, G.; Zehnder, M.; Reis, C.; Fidel, S.; Fidel, R.A.S.; Galan, J.J.; Paciornik, S. Longitudinal Co-Site Optical Microscopy Study on the Chelating Ability of Etidronate and EDTA Using a Comparative Single-Tooth Model. J. Endod. 2008, 34, 71–75. [Google Scholar] [CrossRef]
- Morago, A.; Ordinola-Zapata, R.; Ferrer-Luque, C.M.; Baca, P.; Ruiz-Linares, M.; Arias-Moliz, M.T. Influence of Smear Layer on the Antimicrobial Activity of a Sodium Hypochlorite/Etidronic Acid Irrigating Solution in Infected Dentin. J. Endod. 2016, 42, 1647–1650. [Google Scholar] [CrossRef]
- Tartari, T.; Guimarães, B.M.; Amoras, L.S.; Duarte, M.A.H.; Silva e Souza, P.A.R.; Bramante, C.M. Etidronate Causes Minimal Changes in the Ability of Sodium Hypochlorite to Dissolve Organic Matter. Int. Endod. J. 2015, 48, 399–404. [Google Scholar] [CrossRef]
- Penas, P.P.; Mayer, M.P.A.; Gomes, B.P.F.A.; Endo, M.; Pignatari, A.C.C.; Bauab, K.C.; Pinheiro, E.T. Analysis of Genetic Lineages and Their Correlation with Virulence Genes in Enterococcus Faecalis Clinical Isolates from Root Canal and Systemic Infections. J. Endod. 2013, 39, 858–864. [Google Scholar] [CrossRef]
- Molander, A.; Reit, C.; Dahlén, G.; Kvist, T. Microbiological Status of Root-Filled Teeth with Apical Periodontitis. Int. Endod. J. 1998, 31, 1–7. [Google Scholar] [CrossRef]
- Dezhurko-Korol, V.A.; Novozhilova, N.E.; Makeeva, I.M.; Arkhipova, A.Y.; Moisenovich, M.M.; Akhmadishina, L.V.; Lukashev, A.N.; Semenov, A.M.; Leontieva, M.R.; Byakova, S.F. The Influence of Centrifugation and Inoculation Time on the Number, Distribution, and Viability of Intratubular Bacteria and Surface Biofilm in Deciduous and Permanent Bovine Dentin. Arch. Oral. Biol. 2020, 114, 104716. [Google Scholar] [CrossRef]
- Persoon, I.F.; Crielaard, W.; Özok, A.R. Prevalence and Nature of Fungi in Root Canal Infections: A Systematic Review and Meta-Analysis. Int. Endod. J. 2017, 50, 1055–1066. [Google Scholar] [CrossRef]
- Alshanta, O.A.; Alqahtani, S.; Shaban, S.; Albashaireh, K.; McLean, W.; Ramage, G. Comparison of Three Endodontic Irrigant Regimens against Dual-Species Interkingdom Biofilms: Considerations for Maintaining the Status Quo. Antibiotics 2020, 9, 634. [Google Scholar] [CrossRef]
- Tartari, T.; Wichnieski, C.; Bachmann, L.; Jafelicci, M.; Silva, R.M.; Letra, A.; van der Hoeven, R.; Duarte, M.A.H.; Bramante, C.M. Effect of the Combination of Several Irrigants on Dentine Surface Properties, Adsorption of Chlorhexidine and Adhesion of Microorganisms to Dentine. Int. Endod. J. 2018, 51, 1420–1433. [Google Scholar] [CrossRef]
- Karale, R.; Odedra, K.M.; Srirekha, A.; Champa, C.; Shetty, A.; Pushpalatha, S.; Sharma, R. Effect of Dentin on the Antimicrobial Efficacy of 3% Sodium Hypochlorite, 2% Chlorhexidine, 17% Ethylenediaminetetraacetic Acid, and 18% Etidronic Acid on Candida Albicans: An in Vitro Study. J. Conserv. Dent. 2016, 19, 455–460. [Google Scholar] [CrossRef]
- Arias-Moliz, M.T.; Ordinola-Zapata, R.; Baca, P.; Ruiz-Linares, M.; Ferrer-Luque, C.M. Antimicrobial Activity of a Sodium Hypochlorite/Etidronic Acid Irrigant Solution. J. Endod. 2014, 40, 1999–2002. [Google Scholar] [CrossRef]
- Arias-Moliz, M.T.; Ordinola-Zapata, R.; Baca, P.; Ruiz-Linares, M.; García García, E.; Hungaro Duarte, M.A.; Monteiro Bramante, C.; Ferrer-Luque, C.M. Antimicrobial Activity of Chlorhexidine, Peracetic Acid and Sodium Hypochlorite/Etidronate Irrigant Solutions against Enterococcus Faecalis Biofilms. Int. Endod. J. 2015, 48, 1188–1193. [Google Scholar] [CrossRef]
- Ballal, N.V.; Gandhi, P.; Shenoy, P.A.; Shenoy Belle, V.; Bhat, V.; Rechenberg, D.K.; Zehnder, M. Safety Assessment of an Etidronate in a Sodium Hypochlorite Solution: Randomized Double-Blind Trial. Int. Endod. J. 2019, 52, 1274–1282. [Google Scholar] [CrossRef]
- Pedrinha, V.F.; Cuellar, M.R.C.; Velásquez-Espedilla, E.G.; Duarte, M.A.H.; de Andrade, F.B.; Rodrigues, P.D.A. Impact of Irrigation Protocols with Some Chelators and Mechanical Agitation on Intratubular Decontamination. Braz. Oral Res. 2021, 35, e127. [Google Scholar] [CrossRef]
- Neelakantan, P.; Cheng, C.Q.; Mohanraj, R.; Sriraman, P.; Subbarao, C.; Sharma, S. Antibiofilm Activity of Three Irrigation Protocols Activated by Ultrasonic, Diode Laser or Er:YAG Laser in Vitro. Int. Endod. J. 2015, 48, 602–610. [Google Scholar] [CrossRef]
- Álvarez-Sagües, A.; Herce, N.; Amador, U.; Llinares-Pinel, F.; Nistal-Villan, E.; Presa, J.; Álvarez, L.; Azabal, M. Efficacy of EDTA and HEDP Chelators in the Removal of Mature Biofilm of Enterococcus Faecalis by PUI and XPF File Activation. Dent. J. 2021, 9, 41. [Google Scholar] [CrossRef]
- Giardino, L.; Savadori, P.; Generali, L.; Mohammadi, Z.; Del Fabbro, M.; De Vecchi, E.; Bidossi, A. Antimicrobial Effectiveness of Etidronate Powder (Dual Rinse® HEDP) and Two EDTA Preparations against Enterococcus Faecalis: A Preliminary Laboratory Study. Odontology 2020, 108, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.P.F.A.; Aveiro, E.; Kishen, A. Irrigants and Irrigation Activation Systems In Endodontics. Braz. Dent. J. 2023, 34, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Grawehr, M.; Sener, B.; Waltimo, T.; Zehnder, M. Interactions of Ethylenediamine Tetraacetic Acid with Sodium Hypochlorite in Aqueous Solutions. Int. Endod. J. 2003, 36, 411–417. [Google Scholar] [CrossRef] [PubMed]
- van der Waal, S.; Connert, T.; Laheij, A.; de Soet, J.; Wesselink, P. Free Available Chlorine Concentration in Sodium Hypochlorite Solutions Obtained from Dental Practices and Intended for Endodontic Irrigation: Are the Expectations True? Quintessence Int. 2014, 45, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.P.; Kahler, B.; Walsh, L.J. The Effect of Heating to Intracanal Temperature on the Stability of Sodium Hypochlorite Admixed with Etidronate or EDTA for Continuous Chelation. J. Endod. 2019, 45, 57–61. [Google Scholar] [CrossRef]
- Rossi-Fedele, G.; Guastalli, A.R.; Doğramacı, E.J.; Steier, L.; De Figueiredo, J.A.P. Influence of PH Changes on Chlorine-Containing Endodontic Irrigating Solutions. Int. Endod. J. 2011, 44, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro-Tanomaru, J.M.; Morgental, R.D.; Flumignan, D.L.; Gasparini, F.; Oliveira, J.E.; Tanomaru-Filho, M. Evaluation of PH, Available Chlorine Content, and Antibacterial Activity of Endodontic Irrigants and Their Combinations against Enterococcus Faecalis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 132–135. [Google Scholar] [CrossRef]
- Camps, J.; Pommel, L.; Aubut, V.; Verhille, B.; Satoshi, F.; Lascola, B.; About, I. Shelf Life, Dissolving Action, and Antibacterial Activity of a Neutralized 2.5% Sodium Hypochlorite Solution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2009, 108, e66–e73. [Google Scholar] [CrossRef]
- Mercade, M.; Duran-Sindreu, F.; Kuttler, S.; Roig, M.; Durany, N. Antimicrobial Efficacy of 4.2% Sodium Hypochlorite Adjusted to PH 12, 7.5, and 6.5 in Infected Human Root Canals. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2009, 107, 295–298. [Google Scholar] [CrossRef]
- Zehnder, M.; Kosicki, D.; Luder, H.; Sener, B.; Waltimo, T. Tissue-Dissolving Capacity and Antibacterial Effect of Buffered and Unbuffered Hypochlorite Solutions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2002, 94, 756–762. [Google Scholar] [CrossRef]
- Frough-Reyhani, M.; Ghasemi, N.; Soroush-Barhaghi, M.; Amini, M.; Gholizadeh, Y. Antimicrobial Efficacy of Different Concentration of Sodium Hypochlorite on the Biofilm of Enterococcus Faecalis at Different Stages of Development. J. Clin. Exp. Dent. 2016, 8, e480–e484. [Google Scholar] [CrossRef]
- Alshanta, O.A.; Shaban, S.; Nile, C.J.; McLean, W.; Ramage, G. Candida Albicans Biofilm Heterogeneity and Tolerance of Clinical Isolates: Implications for Secondary Endodontic Infections. Antibiotics 2019, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Ghivari, S.B.; Bhattacharya, H.; Bhat, K.G.; Pujar, M.A. Antimicrobial Activity of Root Canal Irrigants against Biofilm Forming Pathogens—An in Vitro Study. J. Conserv. Dent. 2017, 20, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.-J.; Kim, A.R.; Perinpanayagam, H.; Han, S.H.; Kum, K.-Y. Candida Albicans Virulence Factors and Pathogenicity for Endodontic Infections. Microorganisms 2020, 8, 1300. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Manoil, D.; Näsman, P.; Belibasakis, G.N.; Neelakantan, P. Microbiological Aspects of Root Canal Infections and Disinfection Strategies: An Update Review on the Current Knowledge and Challenges. Front. Oral Health 2021, 2, 672887. [Google Scholar] [CrossRef]
- Arias-Moliz, M.T.; Morago, A.; Ordinola-Zapata, R.; Ferrer-Luque, C.M.; Ruiz-Linares, M.; Baca, P. Effects of Dentin Debris on the Antimicrobial Properties of Sodium Hypochlorite and Etidronic Acid. J. Endod. 2016, 42, 771–775. [Google Scholar] [CrossRef]


| Irrigating Solution | C. albicans | E. faecalis | ||
|---|---|---|---|---|
| Mean (sd) | Median (Q1; Q3) | Mean (sd) | Median (Q1; Q3) | |
| SH | 46.4 (2.8) a | 45 (45; 47) | 12.4 (0.5) A | 12 (12; 13) |
| SH + EDTA | 33.8 (0.8) b | 34 (33; 34) | 12.4 (0.9) A | 13 (12; 13) |
| SH + DR 9% | 51.6 (2.1) c | 52 (50; 53) | 12.4 (0.9) A | 12 (12; 12) |
| SH + DR 15% | 44.2 (0.8) a | 44 (44; 45) | 11.6 (0.5) A | 12 (11; 12) |
| SH + DR 18% | 46.8 (3.3) a | 48 (45; 49) | 12.0 (0.7) A | 12 (12; 12) |
| SH + ID 9% | 46.0 (0.7) a | 46 (46; 46) | 11.6 (0.5) A | 12 (11; 12) |
| SH + ID 15% | 23.2 (3.0) d | 23 (22; 25) | 7.0 (0.7) B | 7 (6; 7) |
| SH + ID 18% | 6.6 (0.9) e | 7 (6; 7) | 6.4 (0.5) B | 6 (6; 7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novozhilova, N.; Babina, K.; Polyakova, M.; Sokhova, I.; Sherstneva, V.; Zaytsev, A.; Makeeva, I.; Mikheikina, A. The Effect of Different Compositions and Concentrations of Etidronate-Containing Irrigants on the Antibacterial Activity of Sodium Hypochlorite against Enterococcus faecalis and Candida albicans. Dent. J. 2024, 12, 46. https://doi.org/10.3390/dj12030046
Novozhilova N, Babina K, Polyakova M, Sokhova I, Sherstneva V, Zaytsev A, Makeeva I, Mikheikina A. The Effect of Different Compositions and Concentrations of Etidronate-Containing Irrigants on the Antibacterial Activity of Sodium Hypochlorite against Enterococcus faecalis and Candida albicans. Dentistry Journal. 2024; 12(3):46. https://doi.org/10.3390/dj12030046
Chicago/Turabian StyleNovozhilova, Nina, Ksenia Babina, Maria Polyakova, Inna Sokhova, Valeria Sherstneva, Alexandr Zaytsev, Irina Makeeva, and Anna Mikheikina. 2024. "The Effect of Different Compositions and Concentrations of Etidronate-Containing Irrigants on the Antibacterial Activity of Sodium Hypochlorite against Enterococcus faecalis and Candida albicans" Dentistry Journal 12, no. 3: 46. https://doi.org/10.3390/dj12030046