Mechanism of Pyrolysis Reaction of Al-Rich Al/PTFE/TiH2 Active Material
Abstract
:1. Introduction
- It has high concentrations of hydrogen. The highest hydrogen storage rate can reach 4%.
- The calorific value of the reaction is high. When the hydrogen content is 3.9%, the calorific value of combustion of titanium hydride is 21.5 MJ/kg [25]. Figure 1 shows the reaction calorific values of TiH2, Al / PTFE and three kinds of high explosives (Trinitrotoluene (TNT), Pentaerythritol tetranitrate (PETN), Cyclotrimethylene trinitramine (RDX)). It can be observed that the energy storage per unit mass of TiH2 is much higher than that of high explosives. Therefore, it has high military value and broad application prospects in the military field.
- The chemical property is stable. It has good compatibility with strong oxidizing substances, long mixed storage time and almost no decomposition. Based on the above advantages, in recent years, some scholars have introduced titanium hydride (TiH2) as a high-energy additive into traditional energetic materials, such as explosives, propellants and pyrotechnics, and carried out a series of related research and made remarkable achievements in terms of explosive explosion performance, propellant burning rate and pyrotechnics explosion performance [26,27,28,29].
2. Materials and Methods
2.1. Materials
2.2. Main Equipment
2.3. Sample Preparation
- According to the mass ratio in Table 3 and Table 4, the powder is placed into the beaker after weighing with the electronic scale. At this time, an appropriate amount of anhydrous ethanol is added into the beaker while continuously stirring for about 30 min, and an approximate fully mixed solution is created. The beaker containing the mixed solution was dried in a vacuum drying oven at 55 °C for 48 h in order to obtain a solid mixture of fully mixed bulk materials.
- The solid mixture of the block material is pounded with a glass rod and continuously stirred into a powder.
2.4. Experimental Content
3. Results and Discussion
3.1. Pyrolysis Behavior and Reaction Process of Polytetrafluoroethylene (PTFE)
3.2. Pyrolysis Behavior and Reaction Process of Titanium Hydride (TiH2)
3.3. Pyrolysis Behavior of Aluminum (Al)
3.4. Pyrolysis Behavior and Reaction Process of Aluminum/Titanium Hydride (Al/TiH2)
3.5. Pyrolysis Behavior and Reaction Process of Polytetrafluoroethylene/Titanium Hydride (PTFE/TiH2)
3.6. Pyrolysis Behavior and Reaction Process of Aluminum/Polytetrafluoroethylene (Al/PTFE)
3.7. Pyrolysis Behavior and Reaction Process of Al-rich Al/PTFE/TiH2
3.8. Combustion Calorific Value Measurement and Chemical Reaction of Active Materials in Oxygen Atmosphere
4. Conclusions
- In argon atmosphere, the pyrolysis of PTFE is an endothermic reaction, and the whole pyrolysis process has only one weightlessness stage, which indicates that the pyrolysis process of PTFE is a one-step reaction. When the temperature is lower than 527 °C, the long chain of PTFE breaks into large molecular weight (C2F4)n and low molecular weight (CF2)n. When the temperature is in the range of 527–596.2 °C, the products of PTFE have large molecular weight (C2F4)n, low molecular weight (CF2)n, C3F6(g), C4F8(g), CF4(g) and C2F6(g). When the temperature is higher than 596.2 °C, the final product of PTFE decomposition is carbon black (C).
- A small endothermic decomposition peak and a larger endothermic decomposition peak appear on the DSC curve of TiH2 under argon atmosphere. The TG curve shows that the total mass loss of sample (TiH2) is 2.97%. Therefore, the pyrolysis of TiH2 is a multistage reaction rather than a one-step reaction. When the temperature is in the range of 386.33–442.9 °C, for the first time, 0.5 hydrogen (H) is removed from TiH2 and converted to TiH1.5, and TiH1.5 is a stable existence in the temperature range of 386.33–470.33 °C. When the temperature is in the range of 470.33–523.8 °C, 1.5 hydrogen is removed from partial TiH1.5 to produce Ti, which coexists with incomplete decomposed TiH1.5. When the temperature is in the range of 650.33–1000 °C, only matter Ti exists.
- In an argon atmosphere, when the temperature is heated from room temperature to 1000 °C, a melting endothermic peak appears on the DSC curve of Al, and the TG curve shows that the mass of the sample (Al) does not change. Therefore, when the temperature is heated from room temperature to 1000 °C, Al only experiences changes in the physical state without chemical changes in matter, namely, solid state → solid–liquid mixed state → liquid state.
- The pyrolysis of Al/TiH2 samples under argon atmosphere is divided into five stages. In the first stage (below 386.33 °C), Al and TiH2 coexist and do not react. In the second stage (386.33–465.1 °C), 0.5 hydrogen (H) is removed from TiH2 for the first time and transformed into TiH1.5, which coexisted with Al. In the third stage (491.3–545.8 °C), 1.5 hydrogen is removed from TiH1.5 to produce Ti, which coexists with Al. In the fourth stage (589.4–671.2 °C), Al reacts with Ti to form Al3Ti, which coexists with partial unreaction of Al and Ti. In the fifth stage (671.2–1000 °C), partial unreaction Al reacts with Ti to form Al2Ti or AlTi, and the disproportionation reaction of Al3Ti occurs to produce Al2Ti and AlTi.
- The pyrolysis of TiH2/PTFE under argon atmosphere can be divided into three stages. In the first stage (328.5–352.5 °C), 0.029 hydrogen (H) was removed from TiH2 and transformed into TiH1.971, then 0.047 hydrogen (H) was removed from TiH1.971 and transformed into TiH1.924, which coexisted with the undecomposed TiH2. In the second stage (532.8–619.7 °C), the endothermic decomposition of PTFE produced C2F4(g), CF4(g), CF2(g), C3F6(g), C4F8(g), C2F6(g) and carbon black (C), while 1.924 hydrogen was completely removed from TiH1.924 and converted into Ti, which reacted with the above gases to produce TiF3 and carbon black (C). In the third stage (619.7–1000 °C), TiC, Ti2CH, C1.04H0.88Ti2, TiC0.957 and Ti8C5 are produced by the mutual reaction of at least two of Ti, C and H.
- The pyrolysis of Al/PTFE under argon atmosphere can be divided into three stages. In the first stage (327.2–351.3 °C), the long chain of partial PTFE breaks into polytetrafluoroethylene with large molecular weight ((C2F4)n) and polytetrafluoroethylene with low molecular weight ((CF2)n), which coexists with Al. In the second stage (527.5–587.8 °C), PTFE decomposes endothermically to produce gases CF4, C3F6, C4F8, C2F6 and carbon black (C), and partial Al reacts with the above gases to generate AlF3 and carbon black (C). In the third stage (650.2–1000 °C), Al reacts with C to form Al4C3, which coexists with partially unreacted Al and AlF3. In addition, it can be observed from the XRD pattern that AlF3 decreases gradually in the temperature range of 650.2–1000 °C.
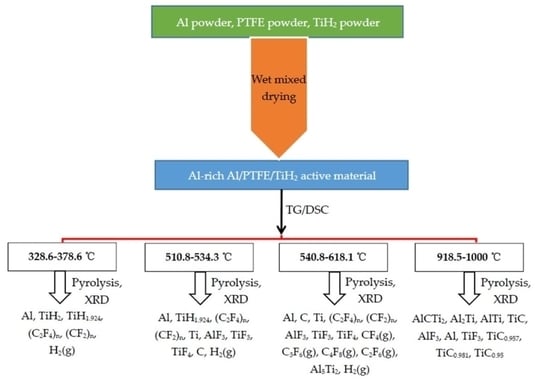
- The pyrolysis of Al-rich Al/PTFE/TiH2 under argon atmosphere can be divided into four stages. In the first stage (328.6–378.6 °C), the long chain fracture of PTFE and dehydrogenation of TiH2 produced TiH1.924, (C2F4)n, (CF2)n and H2(g), which coexist with partially unreacted Al and TiH2. In the second stage (510.8–534.3 °C), 0.076 hydrogen is removed from partially unreacted TiH2 and converted to TiH1.924, and then 1.924 hydrogen is removed from TiH1.924 and converted into Ti. Part of Al and Ti reacts with a part of large and small molecular weight polytetrafluoroethylene to form AlF3, TiF3 and TiF4(g). The main products are Al, TiH1.924, (C2F4)n, (CF2)n, Ti, AlF3, TiF3, TiF4, C and H2(g). In the third stage (540.8–618.1 °C), in addition to polytetrafluoroethylene containing large and small molecular weight, PTFE decomposes endothermically to produce gases CF4, C3F6, C4F8 and C2F6. Al and Ti react with the above gases and polytetrafluoroethylene with molecular weight to produce AlF3, TiF3, TiF4 and carbon black (C). Partial Al reacts with Ti to produce Al5Ti2. The main products are Al, C, Ti, (C2F4)n, (CF2)n, AlF3, TiF3, TiF4, CF4(g), C3F6(g), C4F8(g), C2F6(g), Al5Ti2 and H2(g). In the fourth stage (918.5–1000 °C), at least two elements of Ti, Al and C react with one another to form AlCTi2, Al2Ti, AlTi, TiC, AlF3, Al, TiF3, TiC0.957, TiC0.981 and TiC0.95.
- In composite materials containing two kinds of components, the calorific value of oxygen bomb of Al/TiH2 composite is the largest and is 24,723 J/g, that of which is 28.934% higher than that of Al-rich Al/PTFE. In composite materials containing three kinds of components, the calorific value of oxygen bomb of Al-rich Al/PTFE/TiH2 composite with 10% the content of TiH2 is the largest and is 19,899 J/g. The calorific value of the composites composed of two materials with the same chemical mass ratio increases gradually. With the increase in TiH2 content, the combustion calorific value of oxygen bomb of Al-rich Al/PTFE/TiH2 material first increases and then decreases. Therefore, TiH2 does play a role in increasing the energy of Al-rich Al/PTFE active material, which highlights the role of TiH2 material as a high-energy additive.
- In oxygen atmosphere, the reaction mechanism of various materials is different. For PTFE/TiH2 material, the main products are as follows: (C2F4)n, (CF2)n, CF4(g), C2F6(g), CO2(g), H2(g), C, H2O(g), TiO2, Ti0.912O2, Ti0.924O2, Ti0.992O2, Ti0.936O2 and Ti0.928O2. For the Al/PTFE material, the main products are as follows: C2F4(g), AlF4(g) and CO2(g). For Al/TiH2 material, the main products are as follows: H2(g), H2O(g) and Al2O3. For materials Al-rich Al/PTFE/TiH2-5wt% and Al-rich Al/PTFE/TiH2-10wt%, the two materials have the same products as follows: C2F4(g), H2(g), H2O(g), AlF3, TiF4(g), CO2(g) and Al2O3. For Al-rich Al/PTFE/TiH2-20wt%, the main products are as follows: (C2F4)n(g), H2O(g), AlF3, CO2(g), TiO2, Ti0.72O2, Ti0.784O2 and Al2O3. For Al-rich Al/PTFE/TiH2-30wt%, the main products are as follows: (C2F4)n(g), TiO1.892(OH)0.108, H2O(g), AlF4(g), TiF4(g), CO2(g), TiO2, TiO1.95, Ti0.912O2, Ti0.924O2, Ti0.992O2, Ti0.936O2, Ti0.928O2 and (Al2Ti)O5.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, H.F.; Zheng, Y.F.; Yu, Q.B. Impact-induced initiation and energy release behavior of reactive materials. J. Appl. Phys. 2011, 110, 074904. [Google Scholar] [CrossRef]
- Rose, M.T.; Doll, D.W.; Hodgson, J.R.; Goodell, R.K.; Busky, R.T.; Bray, E.J., II. Reactive Material Enhanced Projectiles and Related Methods. U.S. Patent 2006011086, 19 January 2006. [Google Scholar]
- Mock, W.; Holt, W.H. Impact initiation of rods of pressed polytetrafluoroethylene(PTFE) and aluminum powders. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2006; pp. 1097–1100. [Google Scholar]
- Xu, F.Y.; Geng, B.Q.; Zhang, X.P. Experimental study on behind-plate overpressure effect by reactive material projectile. Propellants Explos. Pyrotech. 2016, 42, 192–197. [Google Scholar] [CrossRef]
- Xu, F.Y.; Zheng, Y.F.; Yu, Q.B. Experimental study on penetration behavior of reactive material projectile impacting aluminum plate. Int. J. Impact Eng. 2016, 95, 125–132. [Google Scholar] [CrossRef]
- Xu, F.Y.; Zheng, Y.F.; Yu, Q.B. Damage effects of aluminum plate by reactive material projectile impact. Int. J. Impact Eng. 2017, 104, 38–44. [Google Scholar] [CrossRef]
- Nielson, D.B.; Tanner, R.L.; Lund, G.K. High Strength Reactive Materials. U.S. Patent 2003096897, 22 May 2003. [Google Scholar]
- Ames, R.G. Energy release characteristics of impact-initiated energetic materials. Dahlgren Div. Nav. Surf. Warface Center 2006, 896. [Google Scholar] [CrossRef]
- Ames, R.G. A standardized evaluation technique for reactive warhead fragments. Int. Symp. Ballist. 2007, 23, 49–58. [Google Scholar]
- Ames, R. Vented chamber calorimetry for impact-initiated energetic materials. AIAA Aerosp. Sci. Meet. Exhib. 2005. [Google Scholar] [CrossRef]
- Lund, G.K.; Nielson, D.B.; Tanner, R.L. High Strength Reactive Materials and Methods of Making. U.S. Patent 2004116576, 17 June 2004. [Google Scholar]
- Kubota, N.; Serizawa, C. Combustion of magnesium/polytetrafluoroethylene. J. Propuls. Power 2012, 3, 303–307. [Google Scholar] [CrossRef]
- Li, L.Q.; Wu, J.B. Explosive properties of the Mg-Al/PTFE composition. Chem. Intermed. 2014, 10, 30–33. [Google Scholar]
- Li, Y.; Jiang, C.L.; Wang, Z.C. Experimental study on reaction characteristics of PTFE/Ti/W energetic materials under explosive loading. Materials 2016, 9, 936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connelljr, T.L.; Risha, G.A.; Yetter, R.A. Boron and polytetrafluoroethylene as a fuel composition for hybrid rocket application. J. Propuls. Power 2015, 31, 1–13. [Google Scholar]
- Ames, R.G.; Brennan, B.P. Measurements of the Effect of Target Skin Thickness on the Impact-Initiated Energy Release from PTFE-Al Projectiles; WBCS Monterey: Monterey, CA, USA, 2003. [Google Scholar]
- Ames, R.G.; Garrett, R.K. Measurements of the Effect of Impact-Initiated Energy Release from Moderate-Density Reactive Materials; WBCS Monterey: Monterey, CA, USA, 2003. [Google Scholar]
- Wang, H.F.; Liu, Z.W.; Yu, W.M. Experimental Investigation of Energy Release Characteristics of Reactive Fragments. Trans. BIT 2009, 29, 663–666. [Google Scholar]
- Zhou, J.; He, Y.; He, Y. Quasi-static compression properties and impact energy release characteristics of Al/PTFE/W reactive materials. Chin. J. Energ. Mater. 2017, 25, 903–912. [Google Scholar]
- Ren, J.K.; Li, Y.C.; Fang, X. Preparation and performance of PTFE/Al/MnO2 composite. Eng. Plast. Appl. 2018, 46, 35–38. [Google Scholar]
- Zhao, P.D.; Lu, F.Y.; Li, J.L. The dynamic compressive properties of PTFE/Al reactive materials. Chin. J. Energ. Mater. 2009, 17, 459–462. [Google Scholar]
- Matijasevic-Lux, B.; Banhart, J.; Fiechter, S. Modification of titaniumhydride forim proved aluminium foam manufacture. Acta Mater. 2006, 54, 1887–1900. [Google Scholar] [CrossRef] [Green Version]
- Zeppelin, F.V.; Hirscher, M.; Stanzick, H. Desorption of hydrogen from blowing agents used for foaming metals. Compos. Sci. Technol. 2003, 63, 2293–2300. [Google Scholar] [CrossRef]
- Li, R.H. Study on the Dynamics Thermal Decomposition of Titaniumhydride; Northeast University: Shenyang, China, 2002. [Google Scholar]
- Li, C.F. Research of raise solid propellant burning rate by using titanium hydride. Winged Missiles J. 1997, 6, 34–37. [Google Scholar]
- Xue, B.; Ma, H.H.; Chen, W. Air Explosion Property of RDX-based Titanium Hydride Composite Explosive. Chin. J. Energ. Mater. 2015, 23, 1046–1050. [Google Scholar]
- Sorensen, D.N.; Quebral, A.P.; Baroody, E.E. Ivestigation of the thermal degradation of the aged pyrotechnic titanium hydride/potassium perchlorate. J. Therm. Anal. Calorim. 2006, 85, 151–156. [Google Scholar] [CrossRef]
- Collins, L.W. The stability and compatibility of TiHx/KClO4 pyrotechnics. J. Hazard. Mater. 1982, 5, 325–333. [Google Scholar] [CrossRef]
- Collins, L.W. Thermal ignition of titanium based pyrotechnics. Combust. Flame 1981, 4, 325–330. [Google Scholar] [CrossRef]
- Yu, Z.S.; Fang, X.; Gao, Z.R. Effect of TiH2 Content on Mechanical Properties and Reaction Characteristics of Al/PTFE Under Quasi-Static Compression. Chin. J. Energ. Mater 2018, 26, 720–724. [Google Scholar]
- Yu, Z.S.; Fang, X.; Li, Y.C. Investigation on the Reaction Energy, Dynamic Mechanical Behaviors, and Impact-Induced Reaction Characteristics of PTFE/Al with different TiH2 Percentages. Materials 2018, 11, 2008. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Yu, Z.S.; Fang, X. Thermal behaviors of Al/TiH2/PTFE ternary active material. Chin. J. Explos. Propellants 2019, 42, 583–596. [Google Scholar]
- Yu, Z.H.; Hao, J.S.; Sun, S.Y.; Shan, C.; Hao, J. PTFE irradiation cleaving and hyperfine powder process. Acta Agric. Nucl. Sin. 2001, 15, 90–93. [Google Scholar]
- Simon, C.M.; Kaminsky, W. Chemical recycling of Polytetrafluoroethylene by pyrolysis. Polym. Degrad. Stab. 1998, 62, 1–7. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Lopez, V.H. The decomposition behavior of as-received and oxidized TiH2 foaming-agent powder. Mater. Sci. Eng. A 2003, 357, 258–263. [Google Scholar] [CrossRef]
- Bhosle, V.; Baburaj, E.G.; Miranova, M.; Salama, K. Dehydrogenation of TiH2. Mater. Sci. Eng. A 2003, 356, 190–199. [Google Scholar] [CrossRef]
- Wei, P.; Zhao, G.Q.; Zhou, Z.Y.; Zhang, C.; Yang, F.; Ding, W. Thermal release behavior of hydrogen in TiHx. Nucl. Tech. 1998, 21, 586–589. [Google Scholar]
- Xin, X.J. Corrosion, Protection and Engineering Application of Titanium; Anhui Science and Technology Press: Hefei, China, 1988. [Google Scholar]
- Atkin, P.W. Physical Chemistry; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Hu, R.Z.; Gang, S.L.; Zhao, F.Q.; Shi, Q.Z.; Zhang, T.L.; Zhang, J.J. Thermal Analysis Kinetics; Science Press: Beijing, China, 2008. [Google Scholar]
- Li, H.M.; Lei, T.; Zhang, J.M.; Fang, S.M.; Shang, Q.-L.; Liu, J. Research on dehydrogenation of TiH2 powder and TiH2 green compact. Mater. Sci. Eng. Pow. Metall. 2012, 17, 270–274. [Google Scholar]
- Li, Y.; Wang, Z.C.; Jiang, C.L.; Niu, H. Experimental study on impact-induced reaction characteristic of PTFE/Ti composites enhanced by W particles. Materials 2017, 10, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.X.; Feng, B.; Gao, Z.R.; Li, Y.C.; Wu, S.Z.; Yin, Q.; Huang, J.; Ren, X. Investigation on the thermal decomposition and thermal reaction process of PTFE/Al/MoO3 fluorine-containing thermite. J. Fluor. Chem. 2021, 241, 109676. [Google Scholar] [CrossRef]























| Main Raw Material Powder | Average Particle Size/(μm) | Purity/(%) | Production Unit |
|---|---|---|---|
| (Polytetrafluoroethylene)PTFE | 27 | >99.5 | HS, Guangdong, China |
| Al | 6–7 | >99.5 | AG, Liaoning, China |
| TiH2 | 4–6 | >99.5 | RF, Hunan, China |
| Anhydrous ethanol | / | 95 | TG, Beijing, China |
| Component | Density/(g/cm3) | Appearance | Melting/Boiling Point(K) | Decomposition Point/(K) | Theoretical ∆H of Reaction with Al/(kJ/g) |
|---|---|---|---|---|---|
| PTFE | 2.200 | White powder | 614/- | 829 | 28.9 |
| Al | 2.702 | Silver grey powder | 933/2600 | - | - |
| TiH2 | 3.910 | Dark grey powder | 673/- | 973 | - |
| NO./Sample | Composition(wt%) | ||
|---|---|---|---|
| Al | PTFE | TiH2 | |
| 1/(PTFE) | / | 100 | / |
| 2/(TiH2) | / | / | 100 |
| 3/(Al) | 100 | / | / |
| 4/(Al/TiH2) | 50 | / | 50 |
| 5/(PTFE/TiH2) | / | 50 | 50 |
| 6/(Al/PTFE/TiH2) | 50 | 50 | / |
| 7/(Al/PTFE/TiH2) | 20 | 50 | 30 |
| NO./Sample | Composition(wt%) | ||
|---|---|---|---|
| Al | PTFE | TiH2 | |
| 1#/(PTFE/TiH2) | / | 50 | 50 |
| 2#/(Al/PTFE) | 50 | 50 | / |
| 3#/(Al/TiH2) | 50 | / | 50 |
| 4#/(Al/PTFE/TiH2) | 45 | 50 | 5 |
| 5#/(Al/PTFE/TiH2) | 40 | 50 | 10 |
| 6#/(Al/PTFE/TiH2) | 30 | 50 | 20 |
| 7#/(Al/PTFE/TiH2) | 20 | 50 | 30 |
| Name of Peak | Initial Temperature/(°C) | Peak Temperature/(°C) | Termination Temperature/(°C) | Enthalpy of Reaction/(J/g) |
|---|---|---|---|---|
| Endothermic peak A | 330.9 | 342.2 | 347.7 | 63.07 |
| Endothermic peak B | 527.0 | 560.1 | 596.2 | 1265 |
| Temperature/(°C) | <527 | 527–596.2 [34] | >596.2 |
|---|---|---|---|
| Decomposition products of PTFE | (CF2)n, PTFE, (C2F4)n | (C2F4)n, C3F6(g), C4F8(g), CF4(g), C2F6(g), (CF2)n | C |
| Name of Peak | Initial Temperature/(°C) | Peak Temperature/(°C) | Termination Temperature/(°C) | Enthalpy of Reaction/(J/g) |
|---|---|---|---|---|
| Endothermic peak A | 386.33 | 442.9 | 470.33 | 109.2 |
| Endothermic peak B | 470.33 | 523.8 | 650.33 | 651.7 |
| Temperature/(°C) | <386.33 | 386.33–650.33 | >650.33 |
|---|---|---|---|
| Decomposition products of TiH2 | TiH2 | TiH1.5, Ti | Ti |
| First Stage | Second Stage | Third Stage | |
|---|---|---|---|
| Temperature/(°C) | 86.33–386.33 | 386.33–470.33 | 470.33–650.33 |
| Initial mass m1/(mg) | 16.6158 | 16.5955 | 16.5002 |
| Termination mass m2/(mg) | 16.5955 | 16.5002 | 16.1223 |
| Loss mass Δm/(mg) | 0.0203 | 0.0953 | 0.3779 |
| Mass loss time/(min) | 59.16362 | 16.7892 | 36.05416 |
| Element | Enthalpy of Decomposition Reaction/(kJ/mol) |
|---|---|
| Ti | |
| H |
| Substance | Heat Capacity Cm/(J/(mol•K)) |
|---|---|
| Ti | 25.02 |
| H2 | 26.88 + 4.347 × 10−3T − 0.3265 × 10−6T2 + 6.656 × 10−9T3 |
| TiH2 | 30.12 |
| Name of Peak | Initial Temperature/(°C) | Peak Temperature/(°C) | Termination Temperature/(°C) | Enthalpy of Reaction/(J/g) |
|---|---|---|---|---|
| Melting endothermic peak A | 654.3 | 661.7 | 670.2 | 299.6 |
| Name of Peak | Initial Temperature/(°C) | Peak Temperature/(°C) | Termination Temperature/(°C) | Enthalpy of Reaction/(J/g) |
|---|---|---|---|---|
| Endothermic peak A | 418.9 | 444.7 | 465.1 | 55.64 |
| Endothermic peak B | 491.3 | 523.9 | 545.8 | 461.3 |
| Exothermic peak C | 598.4 | 633.8 | 671.2 | −389.7 |
| Temperature/(°C) | <386.33 | 386.33–465.1 | 491.3–545.8 | 598.4–671.2 | 671.2–1000 |
|---|---|---|---|---|---|
| Products of the sample (Al/TiH2) | TiH2, Al | TiH1.5, Al, H2(g) | Al, Ti, H2(g) | Al, Ti, Al3Ti | Al2Ti, AlTi |
| Name of Peak | Initial Temperature/(°C) | Peak Temperature/(°C) | Termination Temperature/(°C) | Enthalpy of Reaction/(J/g) |
|---|---|---|---|---|
| Endothermic peak A | 328.5 | 340.6 | 352.5 | 28.62 |
| Exothermic peak B | 532.8 | 554.6 | 571.3 | −565.9 |
| Endothermic peak C | 558.3 | 597.1 | 619.7 | 1006 |
| Temperature/(°C) | 328.5–352.5 | 532.8–619.7 | 619.7–1000 |
|---|---|---|---|
| Products of sample (PTFE/TiH2) | TiH2, TiH1.971, TiH1.924, H2(g) | Ti, TiF3, C, H2(g), C2F4(g), CF2(g) | Ti2CH, C1.04H0.88Ti2, TiC, TiC0.957, Ti8C5 |
| Name of Peak | Initial Temperature/(°C) | Peak Temperature/(°C) | Termination Temperature/(°C) | Enthalpy of Reaction/(J/g) |
|---|---|---|---|---|
| Endothermic peak A | 327.2 | 338.9 | 351.3 | 29.69 |
| Endothermic peak B | 527.5 | 560.8 | 587.8 | 257.4 |
| Endothermic peak C | 650.2 | 660.6 | 671.8 | 128.5 |
| Endothermic peak D | 857.7 | 903.1 | 917.9 | 464.3 |
| Temperature/(°C) | 327.2–351.3 | 527.5–587.8 | 650.2–1000 |
|---|---|---|---|
| Products of sample (PTFE/Al) | Al, (C2F4)n, (CF2)n | Al, (C2F4)n, (CF2)n, CF4(g), C3F6(g), C4F8(g), C2F6(g), C, AlF3 | Al, AlF3, Al4C3 |
| Name of Peak | Initial Temperature/(°C) | Peak Temperature/(°C) | Termination Temperature/(°C) | Enthalpy of Reaction/(J/g) |
|---|---|---|---|---|
| Endothermic peak A | 328.6 | 339.4 | 350.6 | 29.55 |
| Endothermic peak B | 361.4 | 370.1 | 378.6 | 4.615 |
| Exothermic peak C | 510.8 | 523.7 | 534.3 | −22.69 |
| Exothermic peak D | 540.8 | 553.6 | 565.3 | −60.97 |
| Endothermic peak E | 575.5 | 594.5 | 618.1 | 264 |
| Exothermic peak F | 918.5 | 942 | 963.4 | −140 |
| Temperature/(°C) | 328.6–378.6 | 510.8–534.3 | 540.8–618.1 | 918.5–1000 |
|---|---|---|---|---|
| Products of the sample (Al-rich PTFE/Al/TiH2) | Al, TiH2, TiH1.924, (C2F4)n, (CF2)n, H2(g) | Al, TiH1.924, (C2F4)n, (CF2)n, Ti, AlF3, TiF3, TiF4, C, H2(g) | Al, C, Ti, (C2F4)n, (CF2)n, AlF3, TiF3, TiF4, CF4(g), C3F6(g), C4F8(g), C2F6(g), Al5Ti2, H2(g) | AlCTi2, Al2Ti, AlTi, TiC, AlF3, Al, TiF3, TiC0.957, TiC0.981, TiC0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Jiang, C.; Fang, Y.; Wang, X.; Wang, Z. Mechanism of Pyrolysis Reaction of Al-Rich Al/PTFE/TiH2 Active Material. Polymers 2021, 13, 2857. https://doi.org/10.3390/polym13172857
Wang Y, Jiang C, Fang Y, Wang X, Wang Z. Mechanism of Pyrolysis Reaction of Al-Rich Al/PTFE/TiH2 Active Material. Polymers. 2021; 13(17):2857. https://doi.org/10.3390/polym13172857
Chicago/Turabian StyleWang, Yilei, Chunlan Jiang, Yuande Fang, Xinyu Wang, and Zaicheng Wang. 2021. "Mechanism of Pyrolysis Reaction of Al-Rich Al/PTFE/TiH2 Active Material" Polymers 13, no. 17: 2857. https://doi.org/10.3390/polym13172857