A New 3D Iodoargentate Hybrid: Structure, Optical/Photoelectric Performance and Theoretical Research
Abstract
:1. Introduction
2. Results
2.1. Structural Description of Compound 1
2.2. Hirshfeld Surface Analyses of Compound 1
2.3. Characterizations and Optical Behaviors of Compound 1
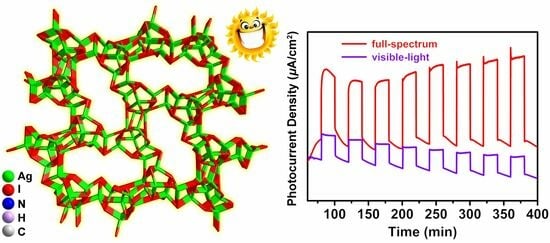
2.4. Photoelectric Performances of Compound 1
2.5. Theoretical Studies of Compound 1
3. Materials and Methods
3.1. Reagents
3.2. Instruments and Measurements
3.3. Preparation of Compound 1
3.4. X-ray Crystallography
3.5. Photoelectric Examinations
3.6. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, T.L.; Guo, Y.M.; Wu, G.X.; Yang, X.F.; Xue, M.; Fu, Y.L.; Wang, M.S. Recent progress of d10 iodoargentate(I)/iodocuprate(I) hybrids: Structural diversity, directed synthesis, and photochromic/thermochromic properties. Coord. Chem. Rev. 2019, 397, 91–111. [Google Scholar] [CrossRef]
- Li, X.X.; Zheng, S.T. Three-dimensional metal-halide open frameworks. Coord. Chem. Rev. 2021, 430, 213663. [Google Scholar] [CrossRef]
- Yu, T.L.; Fu, Y.B.; Wang, Y.L.; Hao, P.F.; Shen, J.J.; Fu, Y.L. Hierarchical symmetry transfer and flexible charge matching in five [M(phen)3]2+ directed iodoargentates with 1 to 3D frameworks. CrystEngComm 2015, 17, 8752–8761. [Google Scholar] [CrossRef]
- Wang, D.; Xue, Z.Z.; Zhang, D.; Pan, J.; Wan, X.Y.; Shao, N.; Guan, Q.W.; Wang, G.M. The iodoargentate framework as a high-performance “sweeper” for specific dye pollutant. Cryst. Growth Des. 2018, 18, 6421–6425. [Google Scholar] [CrossRef]
- Qiao, Y.R.; Hao, P.F.; Fu, Y.L. Symmetrically related construction and optical properties of two noncentrosymmetric 3D iodides of d10 cation (Cu+, Ag+) based on the N-benzylpyridinium and its supramolecular interactions. Inorg. Chem. 2015, 54, 8705–8710. [Google Scholar] [CrossRef]
- Yu, T.L.; Hao, P.F.; Shen, J.J.; Li, H.H.; Fu, Y.L. Stoichiometry-controlled structural and functional variation in two photochromic iodoargentates with a fast and wide range response. Dalton Trans. 2016, 45, 16505–16510. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.L.; Shen, J.J.; Wang, Y.L.; Fu, Y.L. Solvent-dependent iodoargentate hybrids: Syntheses, structural diversity, thermochromism, and photocatalysis. Eur. J. Inorg. Chem. 2015, 11, 1989–1996. [Google Scholar] [CrossRef]
- Zhang, R.C.; Wang, J.J.; Zhang, J.C.; Wang, M.Q.; Sun, M.; Ding, F.; Zhang, D.J.; An, Y.L. Coordination-induced syntheses of two hybrid framework iodides: A thermochromic luminescent thermometer. Inorg. Chem. 2016, 55, 7556–7563. [Google Scholar] [CrossRef]
- Chen, X.; Yao, Z.Y.; Xue, C.; Yang, Z.X.; Liu, J.L.; Ren, X.M. Novel isomorphism of two hexagonal non-centrosymmetric hybrid crystals of M(en)3Ag2I4 (M = transition metal Mn2+ or main-group metal Mg2+; en = ethylenediamine). CrystEngComm 2018, 20, 356–361. [Google Scholar] [CrossRef]
- Li, S.L.; Han, M.; Wu, B.; Wang, J.; Zhang, F.Q.; Zhang, X.M. Observation of contrary thermo-responsive trend for single crystal and powder samples in mechano-, thermo- and solvato-responsive luminescent cubane [Ag4I4L4] cluster. Sci. Rep. 2017, 7, 13058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, W.W.; Sang, L.; Pan, X.W.; Wang, X.Z.; Liu, W.L.; Wang, L.; Ren, X.M. Multi-step structural phase transitions with novel symmetry breaking and inverse symmetry breaking characteristics in a [Ag4I6]2n− cluster hybrid crystal. Chem. Commun. 2020, 56, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Sun, Y.W.; Liu, J.B.; Xu, Q.F.; Zhang, C.Y. [Co(2,2′-bipy)3]Ag3I6 with a hole structure facilitates dye adsorption and photocatalytic reduction. Dalton Trans. 2022, 51, 16784–16789. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Yao, J.; Li, Y.Y.; Xia, Z.R.; Liu, J.B.; Zhang, C.Y. Transition-metal-complex-directed synthesis of hybrid iodoargentates with single-crystal to single-crystal structural transformation and photocatalytic properties. Inorg. Chem. 2020, 59, 13962–13971. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.F.; Wang, W.P.; Zhang, L.F.; Shen, J.J.; Fu, Y.L. Metal-dependent electronic and photochromic behaviors of dimethylbenzotriazolium iodometallate hybrids. Inorg. Chem. Front. 2019, 6, 287–292. [Google Scholar] [CrossRef]
- Sun, C.; Guo, Y.H.; Yuan, Y.; Chu, W.X.; He, W.L.; Che, H.X.; Jing, Z.H.; Yue, C.Y.; Lei, X.W. Broadband white-light emission in one-dimensional organic-inorganic hybrid silver halide. Inorg. Chem. 2020, 59, 4311–4319. [Google Scholar] [CrossRef]
- Lei, X.W.; Yue, C.Y.; Zhao, J.Q.; Han, Y.F.; Yang, J.T.; Meng, R.R.; Gao, C.S.; Ding, H.; Wang, C.Y.; Chen, W.D.; et al. Two types of 2D layered iodoargentates based on trimeric Ag3I7 secondary building units and hexameric Ag6I12 ternary building units: Syntheses, crystal structures, and efficient visible light responding photocatalytic properties. Inorg. Chem. 2015, 54, 10593–10603. [Google Scholar] [CrossRef] [PubMed]
- Song, D.N.; Zhang, D.J.; Wang, Y.L.; Wang, J.J.; Xing, X.S.; Lv, Z.Y.; Liu, F.; Han, J.X.; Zhang, R.C.; Liao, S.J.; et al. Luminescent thermochromic silver iodides as wavelength-dependent thermometers. Inorg. Chem. 2020, 59, 13067–13077. [Google Scholar] [CrossRef]
- Yue, C.Y.; Hu, B.; Lei, X.W.; Li, R.Q.; Mi, F.Q.; Gao, H.; Li, Y.; Wu, F.; Wang, C.L.; Lin, N. Novel three-dimensional semiconducting materials based on hybrid d10 transition metal halogenides as visible light-driven photocatalysts. Inorg. Chem. 2017, 56, 10962–10970. [Google Scholar] [CrossRef]
- Hao, P.F.; Zhang, L.F.; Shen, J.J.; Fu, Y.L. Structural and photochromic modulation of dimethylbenzotriazolium iodoargentate hybrid materials. Dye. Pigment. 2018, 153, 284–290. [Google Scholar] [CrossRef]
- Yu, T.L.; An, L.; Zhang, L.; Shen, J.J.; Fu, Y.B.; Fu, Y.L. Two thermochromic layered iodoargentate hybrids directed by 4-and 3-cyanopyridinium cations. Cryst. Growth Des. 2014, 14, 3875–3879. [Google Scholar] [CrossRef]
- Liu, M.H.; Ren, X.C.; Wen, W.Y.; Li, B.H.; Li, J.Q.; Li, J.; Zhang, B. Three iodoargentate-based hybrids decorated by metal complexes: Structures, optical/photoelectric properties and theoretical studies. Molecules 2023, 28, 6116. [Google Scholar] [CrossRef]
- Jansen, M. Homoatomic d10-d10 interactions: Their effects on structure and chemical and physical-properties. Angew. Chem. Int. Ed. Engl. 1987, 26, 1098–1110. [Google Scholar] [CrossRef]
- Liu, G.N.; Zhang, X.; Wang, H.M.; Xu, H.; Wang, Z.H.; Meng, X.L.; Dong, Y.N.; Zhao, R.Y.; Li, C.C. Do alkyl groups on aromatic or aliphatic structure directing agents affect water stabilities and properties of hybrid iodoargentates? Dalton Trans. 2017, 46, 12474–12486. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hao, P.F.; Shen, J.J.; Fu, Y.L. Two photochromic iodoargentate hybrids with adjustable photoresponsive mechanism. Dalton Trans. 2018, 47, 6031–6035. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Wang, D.; Meng, X.D.; Pan, J.; Han, S.D.; Xue, Z.Z. Construction of iodoargentates with diverse architectures: Template syntheses, structures, and photocatalytic properties. Cryst. Growth Des. 2020, 20, 1130–1138. [Google Scholar] [CrossRef]
- Zhang, R.C.; Wang, J.J.; Yuan, B.Q.; Zhang, J.C.; Zhou, L.; Wang, H.B.; Zhang, D.J.; An, Y.L. Syntheses and characterization of chiral zeolitic silver halides based on 3-rings. Inorg. Chem. 2016, 55, 11593–11599. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Yang, Y.; Wang, W.H.; Shen, H.Y.; Shao, Y.N. A new metal complex-templated silver iodobismuthate exhibiting photocurrent response and photocatalytic property. Dalton Trans. 2022, 51, 13361–13367. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Pang, M.; Wang, Y.S.; Liu, M.Z.; Zhao, H.M. Four discrete silver iodobismuthates/bromobismuthates with metal complexes: Syntheses, structures, photocurrent responses, and theoretical studies. Inorg. Chem. 2022, 61, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, J.; Pang, M.; Chen, X.; Liu, M.Z. Two [Co(bipy)3]3+-templated silver halobismuthate hybrids: Syntheses, structures, photocurrent responses, and theoretical studies. Inorg. Chem. 2022, 61, 9808–9815. [Google Scholar] [CrossRef]
- Li, J.; Liu, M.H.; Shen, H.Y.; Liu, M.Z.; Wu, J.T.; Zhang, B. A semiconductive copper iodobismuthate hybrid: Structure, optical properties and photocurrent response. Dalton Trans. 2023, 52, 2999–3005. [Google Scholar] [CrossRef]
- Creason, T.D.; Fattal, H.; Gilley, I.W.; McWhorter, T.M.; Du, M.H.; Saparov, B. (NH4)2AgX3 (X = Br, I): 1D silver halides with broadband white light emission and improved stability. ACS Mater. Au 2021, 1, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.N.; Jiang, X.M.; Fan, Q.S.; Hussain, M.B.; Li, K.; Sun, H.; Li, X.Y.; Liu, W.Q.; Li, C.C. Water stability studies of hybrid iodoargentates containing N-alkylated or N-protonated structure directing agents: Exploring noncentrosymmetric hybrid structures. Inorg. Chem. 2017, 56, 1906–1918. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.J.; Wang, F.; Li, X.X.; Yu, T.L.; Hao, P.F.; Fu, Y.L. Two photochromic methylated nicotinohydrazide iodoargentate hybrids. Rsc Adv. 2016, 6, 98916–98920. [Google Scholar] [CrossRef]
- Jana, M.K.; Janke, S.M.; Dirkes, D.J.; Dovletgeldi, S.; Liu, C.; Qin, X.X.; Gundogdu, K.; You, W.; Blum, V.; Mitzi, D.B. Direct-bandgap 2D silver-bismuth iodide double perovskite: The structure-directing influence of an oligothiophene spacer cation. J. Am. Chem. Soc. 2019, 141, 7955–7964. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, J. Two hybrid polymeric iodoargentates incorporating aromatic N-heterocycle derivatives as electron acceptors. Inorg. Chem. 2020, 59, 16814–16818. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.W.; Yue, C.Y.; Feng, L.J.; Han, Y.F.; Meng, R.R.; Yang, J.T.; Ding, H.; Gao, C.S.; Wang, C.Y. Syntheses, crystal structures and photocatalytic properties of four hybrid iodoargentates with zero- and two-dimensional structures. CrystEngComm 2016, 18, 427–436. [Google Scholar] [CrossRef]
- Yang, L.L.; Zhou, J.; An, L.T.; Cao, S.M.; Hu, J. A unique formyl iodoargentate exhibiting luminescent and photocurrent response properties. Dalton Trans. 2019, 48, 15762–15766. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Wang, D.H.; Zhao, L.M.; Lin, X.Y.; Wang, Y.K.; Zhang, W.T.; Song, K.Y.; Li, H.H.; Chen, Z.R. Iodoargentate/iodobismuthate-based materials hybridized with lanthanide-containing metalloviologens: Thermochromic behaviors and photocurrent responses. Inorg. Chem. Front. 2018, 5, 1162–1173. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]






| C−H···I | d(C−H) | d(H···I) | d(C···I) | <(CHI) |
|---|---|---|---|---|
| N(1)−H(1)···I(5)#9 | 0.86 | 2.92 | 3.691(12) | 150.5 |
| N(2)−H(2A)···I(3)#4 | 0.86 | 2.87 | 3.718(10) | 170.6 |
| N(3)−H(3)···I(3)#8 | 0.86 | 3.05 | 3.892(12) | 165.7 |
| C(1)−H(1A)···I(6)#9 | 0.93 | 3.05 | 3.903(12) | 153.9 |
| C(4)−H(4)···I(6)#8 | 0.93 | 3.30 | 3.991(13) | 133.2 |
| C(8)−H(8)···I(7)#2 | 0.93 | 3.03 | 3.930(17) | 164.7 |
| C(9)−H(9)···I(2)#8 | 0.93 | 3.07 | 3.873(16) | 145.5 |
| C(10)−H(10)···I(6)#8 | 0.93 | 3.27 | 3.920(15) | 129.0 |
| Compound | D | Template | SG | PBUs | SBUs | I | Reference |
|---|---|---|---|---|---|---|---|
| [H2-4,4′-dpa]Ag6I8 | 3 | [H2-4,4′-dpa]2+ | P21/n | [AgI4] | [Ag6I13] | μ-2; μ-3; μ-4 | This work |
| [N-Bz-Py]4Ag9I13 | 3 | [N-Bz-Py]+ | Cc | [AgI3], [AgI4] | [Ag3I7], [Ag6I12] | μ-2; μ-3; μ-4 | [5] |
| [(Me)2-2,2′-bipy]Ag8I10 | 3 | [(Me)2-2,2′-bipy]2+ | C2/c | [AgI4] | [Ag8I15] | μ-3; μ-4 | [18] |
| [Mg(en)3]Ag2I4 | 3 | [Mg(en)3]2+ | P6322 | [AgI4] | [AgI4] | μ-2 | [9] |
| [Co(phen)3]2Ag13I17 | 3 | [Co(phen)3]2+ | P213 | [AgI4] | [Ag6I13], [Ag7I13] | μ-2; μ-3; μ-4 | [3] |
| [DMBTz]2Ag5I7 | 3 | [DMBTz]+ | C2/c | [AgI4] | [Ag5I9] | μ-1; μ-2; μ-3; μ-4 | [19] |
| [H3(Dabco)2]Ag3I6 | 3 | [H3(Dabco)2]3+ | R32 | [AgI4] | [Ag3I9] | μ-2 | [26] |
| Hmta[(Hmta)Ag4I4] | 3 | Hmta | F-43m | [AgI3] | [Ag4I4] | μ-3 | [8] |
| [(Hmta)2Ag8I6]I2 | 3 | Hmta | Fm-3m | [AgI3] | [Ag8I6] | μ-4 | [8] |
| [emIm]Ag3I4 | 2 | [emIm]+ | Pccn | [AgI4] | [Ag3I8] | μ-2; μ-3 | [17] |
| [MCMP]Ag3I4 | 3 | [MCMP]+ | C2/c | [AgI4] | [Ag6I12] | μ-2; μ-3; μ-4 | [6] |
| [EtPPh3]Ag3I4 | 1 | [EtPPh3]+ | P21/c | [AgI4] | [Ag3I7] | μ-2; μ-3; μ-4 | [23] |
| [MPBI]Ag3I4 | 2 | [MPBI]+ | Pnna | [AgI4] | [Ag3I8] | μ-3 | [24] |
| [DMBTz]Ag3I4 | 1 | [DMBTz]+ | P21/c | [AgI4] | [Ag3I7] | μ-2; μ-3; μ-4 | [14] |
| [Hpy]2Ag6I8·DMF | 2 | [Hpy]+ | Pccn | [AgI4] | [Ag3I8] | μ-3 | [7] |
| [V(DMSO)5(H2O)]Ag6I8 | 2 | [V(DMSO)5]2+ | P21/c | [AgI4] | [Ag5I10] | μ-2; μ-3; μ-4 | [25] |
| Compound 1 | |
|---|---|
| CCDC | 2,301,354 |
| formula | C10H11Ag6I8N3 |
| weight | 1835.64 |
| temperature/K | 298(2) |
| wavelength/Å | 0.71073 |
| crystal system | monoclinic |
| space group | P21/n |
| a/Å | 11.3797(11) |
| b/Å | 14.1621(13) |
| c/Å | 18.8035(17) |
| β/° | 104.387(4) |
| volume/Å3 | 2935.3(5) |
| Z | 4 |
| Dcalcd/g·cm−3 | 4.154 |
| μ/mm−1 | 12.343 |
| F(000) | 3192 |
| reflection collected | 13,260 |
| unique reflection | 5184 |
| Rint | 0.0249 |
| R1 [I > 2s(I)], wR2 [I > 2s(I)] | 0.0415, 0.0955 |
| R1 [all data], wR2 [all data] | 0.0551, 0.1030 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xie, S.; Pang, M.; Zhu, J.; Wu, J.; Zhang, Y.; Zhang, B. A New 3D Iodoargentate Hybrid: Structure, Optical/Photoelectric Performance and Theoretical Research. Molecules 2023, 28, 8033. https://doi.org/10.3390/molecules28248033
Li J, Xie S, Pang M, Zhu J, Wu J, Zhang Y, Zhang B. A New 3D Iodoargentate Hybrid: Structure, Optical/Photoelectric Performance and Theoretical Research. Molecules. 2023; 28(24):8033. https://doi.org/10.3390/molecules28248033
Chicago/Turabian StyleLi, Jun, Shuyue Xie, Ming Pang, Jiacheng Zhu, Jinting Wu, Yongdi Zhang, and Bo Zhang. 2023. "A New 3D Iodoargentate Hybrid: Structure, Optical/Photoelectric Performance and Theoretical Research" Molecules 28, no. 24: 8033. https://doi.org/10.3390/molecules28248033