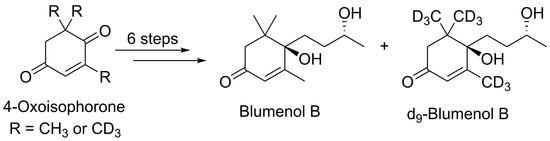
3.2. Synthesis of Compounds
4,4-(Ethylenedioxy)-2,6,6-trimethylcyclohex-2-en-1-one
8: A mixture of 4-oxoisophorone
7 (3.0 g, 19.7 mmol), ethylene glycol (1.46 mL, 26.2 mmol), and toluene-
p-sulfonic acid monohydrate (0.09 g, 0.473 mmol) in toluene (14.8 mL) were heated at reflux overnight with a Dean-Stark trap. The solution was left to cooled and quenched with sat. aq. NaHCO
3 (15 mL) and was extracted with diethyl ether (3 × 15 mL). The combined organic extracts were washed with water and dried over anhydrous MgSO
4. The solvent was removed in vacuo and the crude product was purified by flash chromatography (1:1 hexanes, ethyl acetate) to yield the
title compound 8 (3.325 g, 86%) as a yellow oil.
RF (3:1 petroleum ether, ethyl acetate) = 0.54.
δH (400 MHz; CDCl
3) 1.20 (6H, s, 6-(CH
3)
2), 1.79 (3H, d,
J = 1.39 Hz, 2-CH
3), 2.08 (2H, s, 5-H), 3.98–4.02 (4H, m, OCH
2CH
2O), 6.30 (1H, br s, 3-H). The spectroscopic data were in agreement with the literature values [
31].
4,4-(Ethylenedioxy)-2,6,6-trimethyl-1-trimethylsilanyl-ethynylcyclohex-2-en-1-ol
(±)-9: To a stirred solution of trimethylsilylacetylene (0.29 mL, 2.03 mmol) in THF (3 mL) under an nitrogen atmosphere at −78 °C was added
nBuLi (1.6 M in hexanes, 0.22 mL, 2.03 mmol). The reaction mixture was stirred at −78 °C for 15 min and a solution of
8 (0.20 mg, 1.01 mmol) in THF (6 mL) was added dropwise to the previously prepared reaction mixture. The resultant mixture was then stirred under a nitrogen atmosphere for 1 h, and the reaction was quenched with sat. aq. NH
4Cl (5 mL), and was extracted with diethyl ether (3 × 5 mL), before the combined organic extracts were washed with water (5 mL), brine (5 mL) and dried over anhydrous MgSO
4 and the solvent was removed in vacuo. The crude product was purified by flash chromatography (14:1 hexanes, ethyl acetate) to yield the
title compound (±)-9 (0.22 g, 75%) as a yellow oil.
RF (14:1, 9:1 hexanes, ethyl acetate) = 0.39.
δH (400 MHz; CDCl
3) 0.16 (9H, s, Si(CH
3)
3), 1.05 and 1.12 (each 3H, s, 6-(CH
3)
2), 1.89 (2H, s, 5-H), 1.91 (3H, s, 2-CH
3), 3.90–3.95 (4H, m, 4H, m, OCH
2CH
2O), 5.36 (1H, br s, 3-H). The spectroscopic data were in agreement with the literature values [
31].
4,4-(Ethylenedioxy)-2,6,6-trimethyl-1-ethynylcyclohex-2-en-1-ol
(±)-10: To a stirred solution of
(±)-9 (1.80 g, 6.12 mmol) in methanol (50 mL), potassium carbonate (2.54 g, 18.8 mmol) was added, and the reaction mixture was stirred at room temperature for 1 h. The precipitate was filtered, the solvent was removed in vacuo, and was extracted with diethyl ether (3 × 50 mL); then, the combined organic extracts were washed with water, dried over anhydrous MgSO
4 and the solvent removed in vacuo. The crude product was recrystallised from hexanes to yield the
title compound (±)-10 (1.85 g, 64%) as white needles. R
F (9:1 hexanes, ethyl acetate) = 0.47. Melting point: 76–78 °C (lit. 78–80 °C). δ
H (400 MHz; CDCl
3) 1.10 (3H, s, 6-CH
3), 1.16 (3H, s, 6-CH
3), 1.88 (1H, d,
J = 14.3 Hz, 5-H
a), 1.93 (3H, d,
J = 1.3 Hz, 2-CH
3), 1.96 (1H, d,
J = 14.2 Hz, 5-H
b), 2.02 (1H, s, OH), 2.50 (1H, s, ethynyl CH), 3.90–3.96 (4H, m, OCH
2CH
2O), 5.38 (1H, s, 3-H). The spectroscopic data and melting point were in agreement with the literature values [
31].
8-(3-Hydroxybut-1-yn-1yl)-7,9,9-trimethyl-1,4-dioxaspiro [4.5]dec-6-en-8-ol
(±)-11: To a stirred solution of
(±)-10 (0.85g, 3.8 mmol) in THF (25 mL) under anitrogen atmosphere, LDA (5.71 mL, 11.4 mmol) was added at −78 °C, and the resultant mixture was stirred for 30 min. Acetaldehyde (0.327 mL, 5.85 mmol) in THF (12 mL) was added dropwise to the reaction mixture at −78 °C and was left to stir for 30 min; it was then further stirred at 0 °C under a nitrogen atmosphere overnight. The reaction mixture was quenched with sat. aq. NH
4Cl (15 mL) and extracted with ethyl acetate (3 × 25 mL). The combined organic extracts were washed with brine (25 mL), dried over anhydrous MgSO
4 and the solvent was removed in vacuo. The crude product was purified by flash chromatography (1:1 hexanes, ethyl acetate) to yield the
title compound (±)-10 (0.624 g, 62%) as a white solid. R
F (1:1 hexanes, ethyl acetate) = 0.3. Melting point: 135–138 °C. δ
H (400 MHz; CDCl
3) 1.09 (3H, s, 9-CH
3), 1.14 (3H, s, 9-CH
3), 1.45 (3H, d,
J = 6.5 Hz, CH(OH)C
H3), 1.91 (3H, s, 7-CH
3), 2.03 (2H, br d,
J = 7.6 Hz, 5CH
2), 3.90–3.97 (4H, m, OCH
2CH
2O), 4.54–4.57 (1H, m, C
H(OH)CH
3), 5.36 (1H, br s, 3-H). The
1H NMR spectroscopic data and melting point were in agreement with the literature values [
25].
(±)-Blumenol B
(±)-1: To a stirred solution of
(±)-11 (300 mg, 1.13 mmol) in MeOH (30 mL), Pa/BaSO
4 (120 mg, 40% w/w) was added, and was stirred under a hydrogen atmosphere at room temperature for 18–20 h. The mixture was filtered through Celite
®, washed with MeOH and the solvent was removed in vacuo to yield the alkane (160 mg, 53%) as a colorless oil, which was immediately dissolved in THF (8 mL). The solution was placed under a nitrogen atmosphere, 2 M HCl (0.8 mL) was added, and the resultant mixture was stirred at room temperature overnight. The solvent was removed in vacuo and extracted with ethyl acetate (3 × 5 mL), and the combined organic extracts were washed with brine (5 mL), and dried over anhydrous MgSO
4, and the solvent was
removed in vacuo. The crude product was purified by flash chromatography (1:3 hexanes, ethyl acetate) to yield the
title compound (±)-1 (153 mg, 96%) as a colorless oil. R
F (1:1 hexanes, ethyl acetate) = 0.15. δ
H (400 MHz; CD
3OD) 1.02 (3H, s, 1-H), 1.10 (3H, s, 12-H), 1.22 (3H, d,
J = 6.0 Hz, 10-H), 1.40–1.48 (1H, m, 8-H), 1.68–1.72 (1H, m, 8-H), 1.75–1.83 (1H, m, 7-H), 1.92–1.99 (1H, m, 7-H), 2.04 (3H, s, 13-H), 2.16 (1H, d,
J = 18.1 Hz, 2-H), 2.59 (1H, dd,
J = 4.5, 18.2 Hz, 2-H), 3.66 (1H, qui,
J = 5.7 Hz, 9-H), 5.83 (1H, s, 4-H). δ
C (100 MHz; CD
3OD) 21.8 (C-13), 23.7 (C-10), 24.0 (C-12), 24.5 (C-11), 35.3 (C-8), 35.7 (C-7), 43.0 (C-1), 51.1 (C-2), 69.3 (C-9), 79.2 (C-6), 126.6 (C-4), 171.8 (C-5), 200.8 (C-3). HRMS (ESI+): Found (MNa
+): 249.1457 C
13H
22NaO
3 requires 249.1461. IR: ν
max(film)/cm
−1; 3405 (O-H), 2965 (C-H), 2927 (C-H), 2878 (C-H), 2530 (O-H), 1647 (C=O), 1417 (C=C). Other diastereomer: δ
H (400 MHz; CD
3OD) 1.02 (3H, s, 11-H), 1.10 (3H, s, 12-H), 1.22 (3H, d,
J = 6.0 Hz, 10-H), 1.40–1.48 (1H, m, 8-H), 1.68–1.72 (1H, m, 8-H), 1.75–1.83 (1H, m, 7-H), 1.92–1.99 (1H, m, 7-H), 2.04 (3H, s, 13-H), 2.16 (1H, d,
J = 18.1 Hz, 2-H), 2.59 (1H, dd,
J = 4.5, 18.2 Hz, 2-H), 3.76–3.78 (1H, m, 9-H), 5.88 (1H, s, 4-H). δ
C (100 MHz; CD
3OD) 21.7 (C-13), 23.5 (C-10), 24.0 (C-12), 24.6 (C-11), 35.2 (C-8), 35.6 (C-7), 42.9 (C-1), 51.1 (C-2), 68.9 (C-9), 79.1 (C-6), 126.6 (C-4), 171.7 (C-5), 200.9 (C-3). HRMS (ESI+): Found (MNa
+): 249.1457 C
13H
22NaO
3 requires 249.1461. IR: ν
max(film)/cm
−1; 3405 (O-H), 2965 (C-H), 2927 (C-H), 2878 (C-H), 2530 (O-H), 1647 (C=O), 1417 (C=C). The spectroscopic data were in agreement with the literature values [
25,
29].
(
R)-(But-3-yn-2-yloxy)(
tert-butyl)diphenylsilane
(R)-13: To a stirred solution of (
R)-(+)-3-butyn-2-ol (300 mg, 4.28 mmol) in DMF (15 mL) under an nitrogen atmosphere, imidazole (870 mg, 12.8 mmol) and TBDPSCl (1.66 mL, 6.40 mmol) was added at 0 °C, and was stirred for at room temperature for 4 h. The reaction mixture was quenched with sat. aq. NaHCO
3 (7 mL), and was extracted with ethyl acetate (3 × 15 mL), the combined organic extracts were washed with water (3 × 15 mL), dried over anhydrous MgSO
4 and the solvent was removed in vacuo. The crude product was purified by flash chromatography (9:1 hexanes, ethyl acetate) to yield the
title compound (R)-13 (980 mg, 99%) as a colourless oil. R
F (9:1 hexanes, ethyl acetate) = 0.72. δ
H (400 MHz; CDCl
3) 1.09 (9H, s, Si(CH
3)), 1.40 (3H, d,
J = 6.5 Hz, 1-CH
3), 2.34 (1H, d,
J = 2.5 Hz, 4-H), 4.47 (1H, qd,
J = 6.5, 2.0 Hz, 2-H), 7.36–7.46 (6H, m, Ar-H), 7.60–7.81 (4H, m, Ar-H).
+84.4° (
c = 1.00, MeOH). The spectroscopic data were in agreement with the literature values [
34].
(2
R,3
R)-2,3,7,9,9-Pentamethyl-1,4-dioxaspiro [4.5]dec-6-en-8-one
(R,R)-14: To a stirred solution of 4-oxoisophorone
7 (300 mg, 1.97 mmol) in toluene (8 mL), 2
R,3
R-(+)-2,3-butanediol (236 mg, 2.62 mmol) and
p-toluenesulfonic acid (8.15 mg, 0.0473 mmol) was added. The reaction mixture was heated at reflux for 24 h with a Dean-Stark trap for the removal of excess water. The reaction mixture was cooled and quenched with sat. aq. NaHCO
3 (8 mL), and then extracted with diethyl ether (3 × 8 mL), dried over anhydrous MgSO
4 and the solvent was removed in vacuo. The crude product was purified by flash chromatography (14:1 petroleum ether, ethyl acetate) to yield the
title compound (R,R)-14 (280 mg, 93%) as a pale yellow oil. R
F (3:1 petroleum ether, ethyl acetate) = 0.75. δ
H (400 MHz; CDCl
3) 1.17 (3H, s, 11-H), 1.22 (3H, s, 12-H), 1.27–1.30 (6H, m, 14 and 15-H), 1.79 (3H, d,
J = 1.5 Hz, 13-H), 2.03–2.07 (1H, dd,
J = 14.0, 1.5 Hz, 1H, 10-H), 2.10–2.13 (1H, d,
J = 14.0 Hz, 10-H), 3.61–3.71 (2H, m, 2 and 3-H), 6.33 (1H, t,
J = 1.3 Hz, 6-H).
−16.1 (
c = 0.97, MeOH). The spectroscopic data were in agreement with the literature values [
35].
(2R,3R,8R)-8-((R)-3′((tert-Butyldiphenylsilyl)oxy)but-1′-yn-1′-yl-2,3,7,9,9-pentamethyl-1,4-dioxaspiro [4.5]dec-6-en-8-ol (R,R)-15: To a stirred solution of (R)-13 (288 mg, 0.89 mmol) in THF (8 mL) under an nitrogen atmosphere, and nBuLi (2 M in hexanes, 0.45 mL, 0.89 mmol) was added at −78 °C. The resultant mixture was stirred at −78 °C for 20 min and a solution of (R,R)-14 (100 mg, 0.45 mmol) in THF (4 mL) was added dropwise. The mixture was further stirred under a nitrogen atmosphere at room temperature overnight. The reaction was quenched with sat. aq. NH4Cl (6 mL) and was extracted with diethyl ether (3 × 10 mL), the combined organic extracts were washed with water (6 mL), brine (6 mL) and dried over anhydrous MgSO4 and the solvent was removed in vacuo. The crude product was recrystallised from petroleum ether to yield the title compound (R,R)-15 (112 mg, 47%) as a white solid. Melting point: 95–99 °C. δH (400 MHz; CDCl3) 1.02 (3H, s, 11-H), 1.06 (12H, br s, 12-H and SiC(CH3)), 1.22 (6H, t, J = 5.2 Hz, 16 and 17-H), 1.41 (3H, d, J = 6.5 Hz, 10-H), 1.70 (3H, d, J = 1.35 Hz, 13-H), 1.76 (1H, d, J = 14.1, 2-H), 1.93 (1H, d, J = 14.1, 2-H), 1.86 (2H, dd, J = 59.6, 14.2 Hz, 2-H), 3.50–3.60 (2H, m, 14 and 15-H), 4.52 (1H, q, J = 6.5 Hz, 9-H), 5.30 (1H, s, 4-H), 7.36–7.46 (6H, m, Ar-H), 7.70 (2H, dd, J = 8.0, 2.0 Hz, Ar-H), 7.76 (2H, dd, J = 8.0, 2.0 Hz, Ar-H). δC (100 MHz; CDCl3) 14.3 (C-13), 18.9 (C-16 and 17), 22.3 (C-11 and 12), 25.5 (C-10), 26.9 (SiC(Ph)2C(CH3)3), 39.4 (SiC(Ph)2C(CH3)3), 45.6 (C-2), 60.1 (C-9), 74.3 (C-7), 78.0 (C-14 and 15), 84.1 (C-8), 88.1 (C-6), 104.1 (C-3), 124.8 (C-4), 136.0 (Si(CH3)), 140.4 (C-5). HRMS (ESI+): Found (MNa+): 555.2898 C33H44NaO4Si requires 555.2901.
(R)-6-Hydroxy-6-((R)-9-hydroxybut-7-yn-7-yl)-1,1,5-trimethylcyclohex-4-en-3-one (R,R)-17: To a stirred solution of (R,R)-15 (703 mg, 1.32 mmol) in THF (40 mL) under a nitrogen atmosphere, 2M HCl (6 mL) was added and the mixture stirred under an nitrogen atmosphere at room temperature overnight. The solvent was removed in vacuo and the remaining residue was extracted with ethyl acetate (3 × 40 mL). The combined organic extracts were washed brine (40 mL) and dried over anhydrous MgSO4 and the solvent was removed in vacuo. This crude intermediate (R,R)-16 was dissolved in THF (40 mL) under a nitrogen atmosphere, TBAF (0.73 mL, 2.52 mmol) was added at 0 °C. The resultant mixture was stirred under a nitrogen atmosphere at room temperature overnight. The reaction was quenched with water (40 mL), and the solvent was removed in vacuo, and was extracted with ethyl acetate (3 × 40 mL), the combined organic extracts were washed brine (40 mL) and dried over anhydrous MgSO4 and the solvent was removed in vacuo. The crude product was purified by flash chromatography (1:3 petroleum ether, ethyl acetate) to yield the title compound (R,R)-17 (270 mg, 91%) as a colourless oil. N.B. compound 17 was found to be very volatile and prone to evaporation if placed under strong vacuum. RF (1:1 petroleum ether, ethyl acetate) = 0.23. δH (400 MHz; CDCl3) 1.09 (3H, s, 11-H), 1.19 (3H, s, 12-H), 1.47 (3H, d, J = 6.5 Hz, 10-H), 2.10 (3H, d, J = 1.35 Hz, 13-H), 2.38 (1H, d, J = 16.5 Hz, 2-H), 2.51 (1H, d, J = 16.5 Hz, 2-H), 4.58 (1H, q, J = 6.6, 9-H), 5.83 (1H, s, 4-H). δC (100 MHz; CDCl3) 19.8 (C-12), 22.8 (C-11), 24.4 (C-10), 25.2 (C-13), 29.7 (C-1), 49.4 (C-2), 58.4 (C-9), 74.5 (C-7), 82.8 (C-5), 89.4 (C-8), 126.3 (C-4), 160.4 (C-5), 198.4 (C-3). HRMS (ESI+): Found (MNa+): 245.2712 C13H18NaO3 requires 245.2712. = −138.79° (c = 0.66, MeOH). IR: νmax(film)/cm−1; 3456, 3025, 2864, 1396, 1086.
(6R,9R)-Blumenol B (R,R)-1: To a stirred solution of (R,R)-17 (14 mg, 0.0629 mmol) in MeOH (5 mL), Pd/BaSO4 (1.4 mg, 20% w/w) was added, and the resultant mixture was stirred under a hydrogen atmosphere overnight. The mixture was filtered through Celite®, washed with MeOH and the solvent was removed in vacuo. The crude product was purified by flash chromatography (1:2 petroleum ether, ethyl acetate) to yield the title compound (R,R)-1 (8 mg, 57%) as a colourless oil. RF (1:3 petroleum ether, ethyl acetate) = 0.26. δH (400 MHz; CD3OD) 1.02 (3H, s, 11-H), 1.09 (3H, s, 12-H), 1.16 (3H, d, J = 6.2 Hz, 10-H), 1.40 (1H, dddd, J = 12.7, 11.5, 8.05, 5.12 Hz, 8-H), 1.68 (1H, tt, J = 12.7, 4.5 Hz, 8-H), 1.79 (1H, td, J = 12.7, 4.0 Hz, 7-H), 1.98 (1H, td, J = 12.7, 5.3 Hz, 7-H), 2.04 (3H, d, J = 1.49 Hz, 13-H), 2.16 (1H, dd, J = 18.0, 1.02 Hz, 2-H), 2.59 (1H, d, J = 18.1 Hz, 2-H), 3.61–3.69 (1H, m, 9-H), 5.83 (1H, s, 4-H). δC (100 MHz; CD3OD) 21.8 (C-13), 23.7 (C-10), 24.0 (C-11), 24.5 (C-12), 35.3 (C-7), 35.7 (C-8), 42.9 (C-1), 51.5 (C-2), 69.3 (C-9), 79.2 (C-6), 126.6 (C-4), 171.8 (C-5), 200.8 (C-3). HRMS (ESI+): Found (MNa+): 249.3115 C13H22NaO3 requires 249.3115. = −1.25° (c = 0.16, MeOH). IR: νmax(film)/cm−1; 3469, 3068, 2956, 1410, 1097.
Compounds
d9-19,
d9-20 and
d9-8 were prepared using the reported method [
36].
2,6,6-Tris(methyl-d3)cyclohex-2-ene-1,4-dione-d9 d9-7: To a stirred solution of d9-8 (61 mg, 0.297 mmol) in THF (7 mL) under a nitrogen atmosphere, 2M HCl (3.5 mL) was added, and the resultant mixture was stirred under a nitrogen atmosphere at room temperature overnight. The solvent was removed in vacuo and was extracted with ethyl acetate (3 × 7 mL) and the combined organic extracts were washed with brine (7 mL), and dried over anhydrous MgSO4, and the solvent was removed in vacuo. The crude product was purified by flash chromatography (4:1 petroleum ether, ethyl acetate) to yield the title compound d9-7 (48 mg, quant.) as a colourless oil. RF (3:1 petroleum ether, ethyl acetate) = 0.61. δH (400 MHz; CDCl3) 2.70 (2H, s, 5-H), 6.55 (1H, s, 3-H). δC (100 MHz; CDCl3) 44.9 (C-6), 51.8 (C-5), 137.2 (C-3), 149.0 (C-2), 197.9 (C-4), 203.7 (C-1). HRMS (ESI+): Found (MNa+): 184.2413 C9H3D9NaO2 requires 184.2412. IR: νmax(film)/cm−1; 3049, 2986, 2912, 1768, 1087.
(2R,3R)-2,3-dimethyl-7,9,9-tris(methyl-d3)-1,4-dioxaspiro [4.5]dec-6-en-8-one-d9 d9-(R,R)-14: To a stirred solution of d9-7 (1 g, 6.20 mmol) in toluene (15 mL), a solution of 2S,3S-(+)-2,3-butanediol (0.68 mL, 7.44 mmol) and p-toluenesulfonic acid (37 mg, 0.217 mmol) was added, and the resultant mixture was heated at reflux for 24 h with a Dean–Stark trap for the removal of excess water. The reaction mixture was cooled and quenched with sat. aq. NaHCO3 (15 mL), and was extracted with diethyl ether (3 × 8 mL), dried over anhydrous MgSO4 and the solvent removed in vacuo. The crude product was purified by flash chromatography (14:1 petroleum ether, ethyl acetate) to yield the title compound d9-(R,R)-14 (1.24 g, 89%) as a white solid. RF (9:1 petroleum ether, ethyl acetate) = 0.5. Melting point: 70–76 °C. δH (400 MHz; CDCl3) 1.28 (6H, t, J = 5.45 Hz, 11 and 12-H), 2.02–2.13 (2H, m, 10-H), 3.61–3.71 (2H, m, 2 and 3-H), 6.32 (1H, s, 6-H). δC (100 MHz; CDCl3) 16.7 and 16.9 (C-11 and 12), 47.5 (C-10), 78.5 (C-2 and 3), 103.0 (C-5), 141.5 (C-6), 204.6 (C-8). HRMS (ESI+): Found (MNa+): 256.3423 C13H11D9NaO3 requires 256.3428. + 5.50 (c = 0.4, CHCl3). IR: νmax(film)/cm−1; 3027, 2971, 1695, 1521, 1063.
(6R,14R,15R)-6-((R)-9-((tert-Butyldiphenylsilyl)oxy)but-7-yn-7-yl)-14,15-dimethyl-1,1,5-tris(methyl-d3)-18,19-dioxaspiro [3.19]dec-4-en-6-ol-d9 d9-(R,R)-15: To a stirred solution of (R)-13 (1.62 g, 5.04 mmol) in THF (30 mL) under a nitrogen atmosphere, nBuLi (2 M in hexanes, 2.52 mL, 5.04 mmol) was added at −78 °C. The reaction mixture was further stirred at −78 °C for 20 min and d9-(R,R)-14 (588 mg, 2.52 mmol) in THF (30 mL) was added dropwise. The resultant mixture was then further stirred at room temperature under a nitrogen atmosphere overnight. The reaction was quenched with sat. aq. NH4Cl (30 mL) and was extracted with ethyl acetate (3 × 30 mL), the combined organic extracts were washed with water (30 mL), brine (30 mL) and dried over anhydrous MgSO4 and the solvent was removed in vacuo. The crude product was purified by flash chromatography (9:1 petroleum ether, ethyl acetate) and recrystallised from petroleum ether to yield the title compound d9-(R,R)-15 (1.98 g, 51%) as a white solid. RF (4:1 petroleum ether, ethyl acetate) = 0.41. Melting point: 111–115 °C. δH (400 MHz; CDCl3) 1.05 (9H, s, Si(CH3)), 1.20–1.23 (6H, t, J = 4.8 Hz, 16 and 17-H), 1.41 (3H, d, J = 6.5 Hz, 10-H), 1.52 (1H, br s, OH), 1.74 (1H, d, J = 14.2 Hz, 2-H), 1.92 (1H, d, J = 14.1 Hz, 2-H), 3.50–3.60 (2H, m, 14 and 15-H), 4.52 (1H, q, J = 6.4 Hz, 9-H), 5.28 (1H, s, 4-H), 7.34–7.44 (6H, m, Ar-H), 7.68–7.75 (4H, m, Ar-H). δC (100 MHz; CDCl3) 16.8 (C-16), 16.9 (C-17), 19.2 (2 x CD3), 25.3 (C-10), 26.7 ((-OSi(Ph-C)C(CH3)3)), 26.9 and 39.0 (-OSi(Ph-C)C(CH3)3)), 45.5 (C-2), 60.1 (C-9), 74.3 (C-7), 77.9 and 78.0 (C-14 and 15), 84.1 (C-8), 88.1 (C-6), 104.1 (C-3), 124.8 (C-4), 127.6–136.0 (-OSi(Ph-C)C(CH3)3)), 140.3 (C-5). HRMS (ESI+): Found (MNa+): 564.8435 C33H35D9NaO4Si requires 564.8435. = + 2.27° (c = 0.44, CHCl3). IR: νmax (film)/cm−1; 3574, 3058, 2871, 2133, 1739, 1524, 1088.
(R)-6-((R))-9-((tert-Butyldiphenylsilyl)oxy)but-7-yn-7-yl)-6-hydroxy-1,1,5-tris(methyl-d3)cyclohex-4-en-3-one-d9 d9-(R,R)-16: To a stirred solution of d9-(R,R)-15 (1.98 g, 3.71 mmol) in THF (40 mL) under a nitrogen atmosphere, 2M HCl (10 mL) was added, and the reaction mixture was stirred under anitrogen atmosphere at room temperature overnight. The solvent was removed in vacuo and was extracted with ethyl acetate (3 × 40 mL); the combined organic extracts were washed with brine (40 mL), dried over anhydrous MgSO4 and the solvent was removed in vacuo. The crude product was purified by flash chromatography (3:1 petroleum ether, ethyl acetate) to yield the title compound d9-(R,R)-16 (1.60 g, 94%) as white crystals. RF (3:1 petroleum ether, ethyl acetate) = 0.51. Melting point: 105–107 °C. δH (400 MHz; CDCl3) 1.05 (9H, s, Si(CH3)), 1.46 (3H, d, J = 6.5 Hz, 10-H), 2.25 (1H, d, J = 16.5 Hz, 2-H), 2.34 (1H, d, J = 16.5 Hz, 2-H), 4.57 (1H, q, J = 6.5 Hz, 9-H), 5.73 (1H, s, 4-H), 7.36–7.46 (6H, m, Ar-H), 7.70 (4H, dt, J = 20.4, 6.1, 1.5 Hz, Ar-H). δC (100 MHz; CDCl3) 19.2 (C-1), 25.2 (C-10), 26.8 ((-OSi(Ph-C)C(CH3)3)), 41.2 (C-2), 59.9 (C-9), 74.2 (C-6), 82.4 (C-8), 89.7 (C-7), 126.1 (C-4), 127.7 and 127.9 (-OSi(Ph-C)C(CH3)3)), 130.0 (-OSi(Ph-C)C(CH3)3)), 135.8 and 136.0 (-OSi(Ph-C)C(CH3)3)), 160.0 (C-5), 198.3 (C-3). HRMS (ESI+): Found (MNa+): 492.2878 C29H27D9NaO3Si requires 492.2891. = −2.35° (c = 0.34, CHCl3). IR: νmax(film)/cm−1; 3369, 3056, 2863, 1786, 1121.
(R)-4-Hydroxy-4-((R)-3′-hydroxybut-1-yn-1-yl)-3,5,5-tris(methyl-d3)cyclohex-2-en-1-one-d9 d9-(R,R)-17: To a stirred solution of d9-(R,R)-16 (69 mg, 0.15 mmol) in THF (7 mL) under a nitrogen atmosphere, TBAF (0.09 mL, 0.30 mmol) was added at 0 °C, and the resultant mixture was stirred at room temperature under a nitrogen atmosphere overnight. The reaction was quenched with water (7 mL), the solvent was removed in vacuo and extracted with ethyl acetate (3 × 7 mL), the combined organic extracts were washed with brine (7 mL) and dried over anhydrous MgSO4 and the solvent was removed in vacuo. The crude product was purified by flash chromatography (1:2 petroleum ether, ethyl acetate) to yield the title compound d9-(R,R)-17 (35 mg, quant.) as a colourless oil. N.B. compound 17 was found to be very volatile and was prone to evaporation if placed under a strong vacuum. RF (1:1 petroleum ether, ethyl acetate) = 0.26. δH (400 MHz; CDCl3) 1.46 (3H, d, J = 6.5 Hz, 10-H), 2.37 (1H, d, J = 16.4 Hz, 2-H), 2.49 (1H, d, J = 16.4 Hz, 2-H), 2.83 (OH), 4.58 (1H, q, J = 6.6 Hz, 9-H), 5.83 (1H, s, 4-H). δC (100 MHz; CDCl3) 24.4 (C-10), 49.2 (C-2), 58.4 (C-9), 74.4 (C-6), 82.7 (C-7), 89.3 (C-8), 126.2 (C-4), 198.5 (C-3). HRMS (ESI+): Found (MNa+): 254.1704 C13H9D9NaO3 requires 254.1713. = −150.51° (c = 0.78, MeOH). IR: νmax(film)/cm−1; 3594, 2861, 2236, 1811, 1067.
(6R,9R)-Blumenol B-d9 d9-(R,R)-1: To a stirred solution of d9-(R,R)-17 (54 mg, 0.233 mmol) in MeOH (7 mL), Pd/BaSO4 (10 mg, 20% w/w) was added, and was stirred under a hydrogen atmosphere for 6 h. The mixture was filtered through Celite®, washed with MeOH and the solvent was removed in vacuo. The crude product was purified by flash chromatography (1:3 petroleum ether, ethyl acetate) to yield the title compound d9-(R,R)-1 as (24 mg, 44%) as a colourless oil. RF (1:3 petroleum ether, ethyl acetate) = 0.24. δH (400 MHz; CD3OD) 1.17 (3H, d, J = 6.2 Hz, 10-H), 1.34–1.44 (1H, m, 8-H), 1.64–1.82 (2H, m, 8-H), 1.94–2.02 (1H, m, 7-H), 2.15 (1H, dd, J = 18.1, 0.98 Hz, 2-H), 2.59 (1H, d, J = 18.1 Hz, 2-H), 3.62–3.69 (1H, m, 9-H), 5.83 (1H, s, 4-H). δC (100 MHz; CD3OD) 23.7 (C-10), 35.3 (C-7), 35.7 (C-8), 50.9 (C-2), 69.4 (C-9), 79.2 (C-6), 126.6 (C-4), 171.7 (C-5), 200.9 (C-3). HRMS (ESI+): Found (MNa+): 258.2018 C13H13D9NaO3 requires 258.2026. = −2.272° (c = 0.22, CHCl3). IR: νmax(film)/cm−1; 3412.1 (broad OH), 2850.9, 2917.2 and 2961.0 (C-H), 1650.9 and 1729.3 (C=O), 1275.4 (C-O).