Research Thesis: Light-assisted thermal catalysis of carbon dioxide methanation enhancement by lanthanide incorporation over cobalt-alumina(Co/Al2O3)
Abstract
The continuous increase in CO2 concentrations in the atmosphere have put a great importance to convert it to useful products. The conversion of CO2 to methane (CH4), which is called the Sabatier reaction, offers a promising alternative but faces kinetic limitations due to heating requirements. The heating requirement can be, in part, offset by using light to boost catalyst performance.
Co/Al2O3 catalyst has been widely investigated along with many catalysts and has shown excellent catalytic performance for the CO2 methanation reaction. However, catalysts can be further enhanced by incorporating promotors such as rare earth metals. A previously conducted study within the PartCat Research Group demonstrated that incorporating lanthanum into Co/Al2O3 improved the light assisted catalysis for the methanation reaction. The research conducted in my study expanded on this result by examining the effect of doping the Co/Al2O3 with other lanthanides on the catalytic methanation reaction under both non-illuminated (dark) and illuminated (light) conditions. Herein, a series of lanthanide doped catalysts (Ln-Co/Al2O3) were prepared using Double Flame Spray Pyrolysis (DFSP). The lanthanides investigated were La, Pr, Eu, and Ce at a 10wt% loading in a 10wt%Co on alumina catalyst (10Ln-10Co-80Al2O3). N2 adsorption- desorption results showed there was an insignificant decrease in BET surface area upon the incorporation of the lanthanides. XRD results revealed an absence of Co3O4 diffraction peaks upon the lanthanide promoted catalysts which indicate that Co particles are highly dispersed over the catalyst surface.
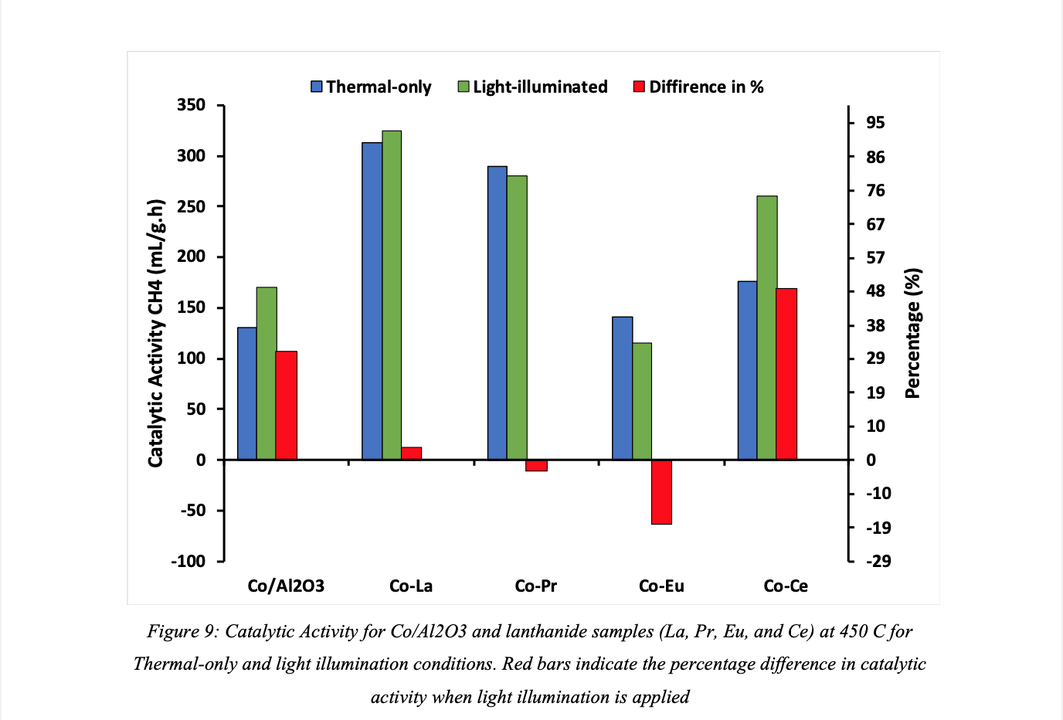
Activity tests revealed that the La, Pr and Ce promoted catalysts improved catalytic performance such that CO2 conversion slightly increased by 5%, 3%, and 1% for Pr- Co/Al2O3, La- Co/Al2O3, and Ce- Co/Al2O3, respectively, compared to bare Co/Al2O3 catalyst under thermal-only conditions. However, CH4 selectivity was enhanced upon the addition of lanthanides in the order La>Pr>Ce which suggest a relation to surface basicity, but further characterisation is required to confirm. In contrast, a negative effect was observed upon the addition of Eu such that CO2 conversion was decreased when compared to neat Co/Al2O3.
Light illumination was found to enhance the catalytic performance of La and Ce promoted catalysts such that catalytic activity was increased by nearly 50% upon Ce incorporation and 4% for La promoted catalyst. Neat Co/Al2O3 experienced a 31% increase in catalytic activity for the illuminated case which suggest that lanthanide incorporation has a minimal effect in increasing catalytic activity for light assisted catalysis. However, it was observed that Pr and Eu promoted catalysts experienced a negative effect under light illumination as CO2 conversion decreased for temperatures above 300 °C.
Recommendations for future plans were suggested which include the investigation of lanthanide incorporation and the increase surface basicity and active sites which can be evaluated using CO2 TPD and NH3 Temperature Programmed Desorption studies. Further, conducting in-situ DRIFTS studies will provide an insight on the intermediary species involved in CO2 adsorption on the surface of the catalyst which is linked to the lanthanide incorporation under light illumination. Also, conducting X-ray Photoelectronic Spectroscopy (XPS) will provide an insight on the catalyst-support interaction. Furthermore, Transmission Electron Microscopy (TEM) analysis and UV-vis spectrometry are recommended to provide an understanding about the photostability and light absorbance properties respectively for different promoted catalyst under light illumination.
If you are interested in reading the whole research paper please reach out through DM.