The ATX(N) classification system and its potential use in clinical practice and therapy development for Alzheimer’s disease
Alzheimer’s disease (AD) is a devastating neurodegenerative disease. It is estimated to affect around 43.8 million individuals and ranks as the fifth leading cause of death globally [1]. Historically, the diagnosis of AD during life was based on clinical symptoms [2]. Such an approach has significant limitations; first, AD-like symptoms are not always caused by AD pathophysiology; second, diagnostic frameworks based on clinical symptoms are not suitable for multifactorial and chronic diseases like AD that have long preclinical or prodromal stages—a critical time window for early intervention [3].
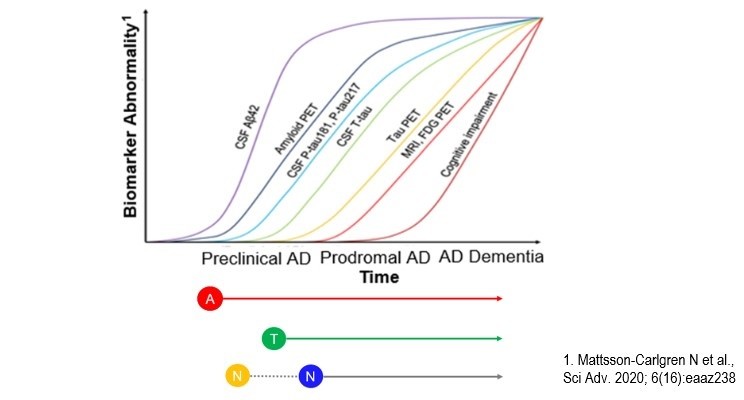
Concerning the latter point, the last three decades have seen significant progress in the development and validation of fluid and neuroimaging biomarkers charting AD pathophysiological alterations, which has catalyzed the conceptual transformation of AD to a clinical-biological construct [4]. In this context, a biomarker-driven classification system called the AT(N) system was proposed recently to classify individuals in an unbiased “symptoms-agnostic” fashion based on key AD-related pathophysiological changes—the amyloid-β pathway (‘A’), tau pathology (‘T’), and neuronal injury/neurodegeneration (‘N’). The system is flexible and expected to evolve to an ATX(N) system, where X stands for additional pathophysiological mechanisms that are relevant for AD.
During the virtual AAIC meeting this year, Eisai hosted a medical symposium entitled “The Development of the ATX(N) Classification System for Different Contexts of Use Across the Alzheimer’s Disease Continuum: State-of-the-art and Future Perspectives for Clinical Practice and Therapy Development.” A top-level faculty of world-class thought leaders in the field contributed to this project, including Dr. Jeffrey Cummings, a world-renowned leader in the development of new therapies for AD; Dr. Clifford Jack, a leading expert in clinical imaging research in AD; and Dr. Kaj Blennow, a world leader in fluid biomarker development for Alzheimer’s and other neurodegenerative diseases.
Dr. Cummings gave an overview of the AT(N) system and summarized the current neuroimaging, cerebrospinal fluid (CSF), and blood biomarkers of the framework. The AT(N) framework provides a guide to the biological investigation of AD, and the contexts of use include screening, diagnosis, prognosis, target engagement (in clinical trials), disease modification, and treatment monitoring. As a specific example, a study showed that in individuals with clinical presentation of mild cognitive impairment due to AD or mild AD dementia respectively, 50% and 25%, in fact, did not have amyloid-β (Aβ) pathology [5]. Such individuals should not be included in AD therapy trials since other non-AD pathologies likely underlie the clinical symptoms. This is just one of the examples on how the AT(N) system can be used to better design and conduct clinical trials.
Dr. Jack described the history and rationale for developing the AT(N) system. The various diagnostic guidelines in the mid-2010s contained disagreements on staging, terminology, and interpretation of biomarkers, which created uncertainty for investigators, regulators, and industry partners. The AT(N) concept was formulated in 2016 to provide a unified conceptual approach to categorize AD biomarkers at the individual level in a format that is easy to understand and use [6]. Dr. Jack also provided several use cases for the AT(N) system. In older adults without dementia, those with positive A biomarkers combined with either T or N biomarkers (or both) exhibited significantly faster memory decline in the next five years than those with other AT(N) profiles [7]. These results suggest that the AT(N) system can have prognostic value in clinical practice.
Dr. Blennow provided a comprehensive overview of the development and validation of CSF and blood biomarkers of the AT(N) system. Recent progress in AD blood biomarker development, such as plasma Aβ42/40 [8], p-Tau181 [9-11], and p-Tau217 [12], is particularly exciting. Once developed and validated in relevant clinical populations, a blood-based AT(N) system could enable large-scale biological screening and diagnostic-therapeutic decision-making, and represents a globally accessible and sustainable solution for the next-generation AD patient journey.
To watch the three presentations and learn more about the AT(N) system, please click here.
This article was written and approved by Eisai Inc.
References
1 Collaborators, G. B. D. D. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18, 88-106, doi:10.1016/S1474-4422(18)30403-4 (2019).
2 McKhann, G. et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34, 939-944, doi:10.1212/wnl.34.7.939 (1984).
3 Jack, C. R., Jr. et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7, 257-262, doi:10.1016/j.jalz.2011.03.004 (2011).
4 Jack, C. R., Jr. et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement 14, 535-562, doi:10.1016/j.jalz.2018.02.018 (2018).
5 Sevigny, J. et al. Amyloid PET Screening for Enrichment of Early-Stage Alzheimer Disease Clinical Trials: Experience in a Phase 1b Clinical Trial. Alzheimer Dis Assoc Disord 30, 1-7, doi:10.1097/WAD.0000000000000144 (2016).
6 Jack, C. R., Jr. et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87, 539-547, doi:10.1212/WNL.0000000000002923 (2016).
7 Jack, C. R., Jr. et al. Associations of Amyloid, Tau, and Neurodegeneration Biomarker Profiles With Rates of Memory Decline Among Individuals Without Dementia. JAMA 321, 2316-2325, doi:10.1001/jama.2019.7437 (2019).
8 Nakamura, A. et al. High performance plasma amyloid-beta biomarkers for Alzheimer's disease. Nature 554, 249-254, doi:10.1038/nature25456 (2018).
9 Janelidze, S. et al. Plasma P-tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med 26, 379-386, doi:10.1038/s41591-020-0755-1 (2020).
10 Karikari, T. K. et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 19, 422-433, doi:10.1016/S1474-4422(20)30071-5 (2020).
11 Thijssen, E. H. et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med 26, 387-397, doi:10.1038/s41591-020-0762-2 (2020).
12 Palmqvist, S. et al. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA 324, 772-781, doi:10.1001/jama.2020.12134 (2020.)