
Adhesion
n., plural: adhesions
[ˌædˈhiː.ʒən]
Definition: ability of a structure or substance to stick to another of not the same kind, such as a cell binding to a substrate
Table of Contents
Adhesion Definition
Adhesion is the binding or attraction between dissimilar molecules, atoms, surfaces, or substances. The adhesive forces that bind two dissimilar substances or surfaces are electrostatic forces and mechanical forces. Examples of adhesion include the capillary action of a liquid and the formation of a tissue due to the adhesion of different types of cells.
In general science, adhesion means the sticking of molecules or surfaces to each other.
In cell biology, adhesion is the binding of two or more cells together or the binding of a cell to a surface through cell adhesion molecules (CAM). CAM molecules hold the cells together thus forming tissues. Cell adhesion is a fundamental phenomenon for holding parts united in a multicellular organism.
Adhesion is a surface phenomenon. Adhesion is the outcome of attractive forces that bind together dissimilar or different molecules. Thus, in biology, adhesive forces can hold two surfaces together. Similarly, in chemistry, it will enable two dissimilar molecules or atoms to bind together, and in physics, it will help to hold together two dissimilar substances. To simply understand it, adhesives, like glue or cement, help to bind different surfaces together.
Here it is important to understand that the binding of similar molecules or surfaces is known as cohesion. Thus, cohesion is an intramolecular phenomenon that holds together the bulk of a substance (Figure 1).
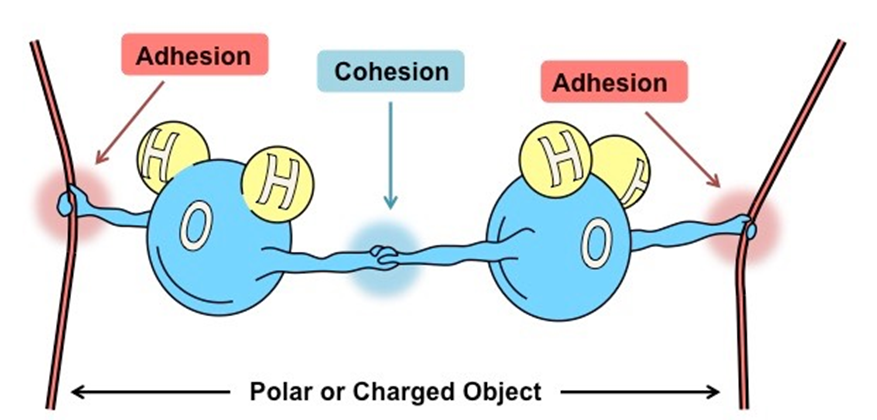
Cohesion represents the atomic forces that hold the bulk of a substance whereas adhesion involves the attractive forces at the interface of the two surfaces. Electrostatic forces are responsible for the adhesion whereas Van der Waal’s forces result in cohesive forces.
Adhesion forces can be between solid-solid and solid-liquid. The wettability of a liquid is attributed to the adhesive forces, i.e., a liquid/water sticking to a solid surface like water molecules on the surface of leaves is an example of adhesion.

Adhesion is the binding of a cell to another cell or a cell to a surface through cell adhesion molecules. An example is a malarial protozoan cell (Plasmodium falciparum) binding to a liver cell via the cell adhesion molecule called the circumsporozoite protein. In anatomy, adhesion refers to the binding of fibrous bands that form between tissues and organs, often as a result of injury during surgery. An example is interthalamic adhesion, which is a band connecting the two thalami of the brain. In general, adhesion refers to the joining of different or dissimilar substances or the attraction between the surfaces of contacting bodies. For instance, cohesion causes water to form drops and adhesion keeps the water drops on the surfaces of leaves and flowers in place.
Etymology: Latin adhaesiō, adhaesiōn-, from “adhaesus”, past participle of adhaerēre (to adhere). The literal meaning of adhesion: ‘ad’, meaning “other” and ‘hesion’, meaning “to stick”. Thus, adhesion means sticking to others (just as allegiance or faithfulness to an idea, a person, or a political party).
Compare: cohesion
-
Adhesion vs. Cohesion
Let us now differentiate between adhesion and cohesion.
Table 1: Adhesion vs Cohesion |
|
|---|---|
| Adhesion | Cohesion |
| It is a surface phenomenon that is seen between dissimilar molecules or surfaces | It is a phenomenon seen between two similar molecules or atoms |
| It is an intermolecular phenomenon | It is an intramolecular phenomenon |
| Electrostatic forces and mechanical forces are the attractive forces that result in adhesion | Van der Waal’s forces and hydrogen bonding are the attractive forces acting in cohesion |
| Adhesive forces are responsible for the spreading of water or liquid over a surface | Cohesive forces are responsible for the formation of water droplets |
| Capillary action of water and formation of the meniscus involve adhesive forces | Capillary action, surface tension, and water meniscus involve cohesive forces |
Data Source: Dr. Amita Joshi of Biology Online
Watch this vid about the difference between Adhesion and cohesion :
Adhesion in Biology
Some common examples of adhesion in the biological sciences are listed below.
-
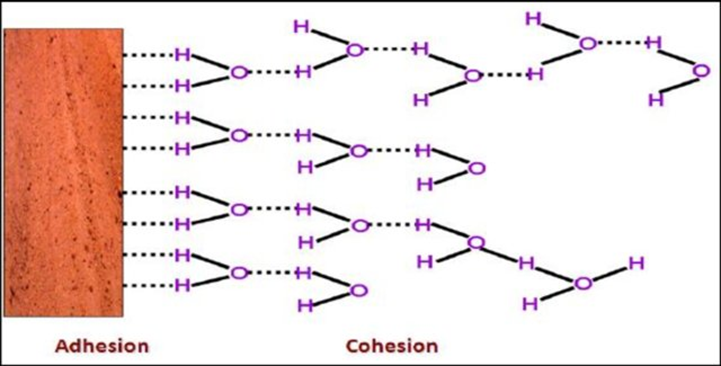
Spreading of water molecules
A water molecule is a polar molecule wherein one part of the molecule possesses a positive charge and the other part is negatively charged. Since opposite charges attract, the water molecule is capable of binding with other polar molecules resulting in the adhesion and spreading of water molecules to surfaces and other molecules. (Interestingly, due to their polar nature, water molecules are attracted to each other, too, resulting in their cohesion.) (Figure 3)
-
Capillary Action
Capillary action is also referred to as capillary effect, capillary rise, capillarity, or capillary motion. Capillary action is the process of movement of a liquid in the upward direction in a narrow space against gravity without any external assistance for it. The most common example of capillary action is the movement of water in a wick to completely make it wet when dipped in water at one end only. The intermolecular adhesion forces between the liquid and the solid surface are the reason for the capillary movement of liquid. However, it is important to note that capillary action is an interplay of adhesive as well as cohesive forces (Figure 4).
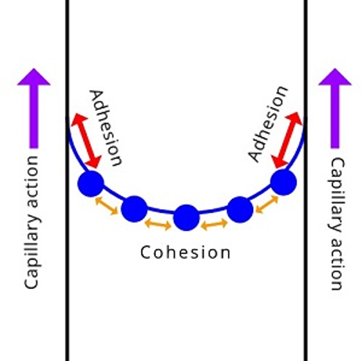
The combination of adhesive and cohesive forces propels the liquid on the solid surface against gravity. The movement of the water in plants and trees to travel from roots to different parts of the plant or a tree occur due to capillary action. In plants, water transportation occurs in xylem vessels.
Xylem vessels are made up of cellulose. Adhesive forces between water molecules and cellulose propel water molecules through the xylem to different parts of the plants. Cohesive forces also work in conjunction with adhesive forces for water transportation through the xylem in plants. A dynamic balance between cohesive forces and adhesive forces is responsible for the water movement through capillary action.
-
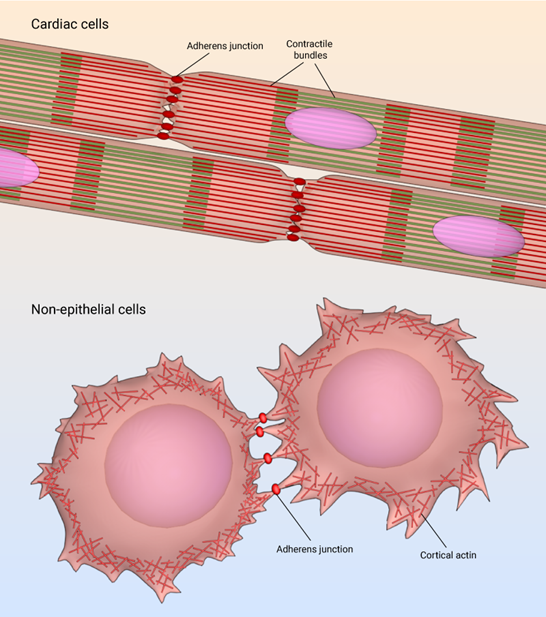
Cell-to-cell adhesion
The formation of tissue involves close interaction between different kinds of cells. Adhesive forces between different types of cells help to form a tissue (Figure 5). Thus, cell adhesion is the fundamental process in multicellular organisms. Cell adhesion molecules (CAM) on each cell help adhesion of different cells as well as their interaction with extracellular fluid. All the cellular processes like cell signaling, cell migration, homeostasis, and development of tissue, occur due to cell adhesion.
Cell adhesion also is affected in different disease conditions, for example., in cancer. In cancer, cell adhesion is dysregulated. Cancer cells are generally loosely attached and because of this these cells detach easily and travel through the blood to cause metastasis of cancer. CAM molecules have been found to be dysregulated in cancer.
Note it!
Cell-to-cell adhesion occurs through four major adhesion molecules, namely, Cell Adhesion Molecules (CAM), Selectins, Cadherins, and Integrins.
Adhesion in Anatomy
In anatomy, adhesion is the abnormal union of tissues as an outcome of an inflammatory process or the formation of a fibrous band of scar tissue band that holds or sticks together the organs that are usually separated. Such adhesions are most commonly seen after abdominal surgery, post which an abdominal adhesion as a scar is seen between abdominal organs.
Another common adhesion seen in women is intrauterine adhesions. However, such adhesions are less commonly seen after laparoscopic surgery. Hence, laparoscopic interventions, wherever possible, are preferred to reduce the risk of new adhesions as abdominal surgery has increased the risk of organ adhesion. Detection of such organ adhesions cannot be detected with the help of blood tests. Imaging techniques are used for the diagnosis of adhesions.
Mechanism of Adhesion
Adhesive properties are an outcome of an either-or combination of different mechanisms. Broadly, the adhesive strength of substances and adhesion is the outcome of either of the following mechanism:
-
Mechanical interlocking
Mechanical forces are one of the major forces involved in adhesion. This usually comes into the picture when one of the surfaces is uneven wherein the other substance, usually a liquid, can anchor or penetrate into it resulting in proper adhesion. In such a case the viscosity of the liquid and cavity shape will determine the efficiency with which liquid will fill in the surface cavities of the uneven surface. A well-filled cavity will ensure strong adhesive forces between the two dissimilar surfaces i.e., solid and liquid.
-
Chemical bonding
Two surfaces, if capable of forming chemical bonds, will result in the adhesion of two molecules or surfaces. Chemical bonding, ionic or covalent, results in one of the strongest bonds between two molecules, while in comparison, the formation of the hydrogen bond is a relatively weaker form of adhesion between two surfaces or molecules. Hydrogen bonds are formed wherein the hydrogen atom of one molecule forms a bond with oxygen/fluoride/nitrogen present in another molecule. However, ionic and covalent bonds are effective over a short distance only (~<1nm) and hence, the two surfaces/molecules should be in close contact with each other to form such bonds.
-
Dispersive adhesion
It is also referred to as physisorption, wherein Van der Waal’s forces play a role in adhesion. Van der Waals forces are the weak forces acting between two surfaces or molecules having slight polarity as compared to the bulk of the material i.e., slightly positive and negative. A large surface or molecule can have multiple regions of polarity.
The polarity of the surface or molecule may be a temporary property (keesom forces) or it may be transient (London forces).
In physical chemistry or general science, adhesion is primarily used for dispersive adhesion.
- Solid-liquid-gas adhesion, for eg., a drop of liquid on a solid surface in the air, can be measured
- Indirect measurement of Adhesion- by measuring the Contact angle. The contact angle is inversely proportional to the adhesiveness i.e., a lower contact angle indicates higher adhesiveness or wettability. Although, the contact angle is an outcome of both adhesive forces as well as cohesive forces as specified below in Table 2.
- Direct measurement of Adhesion- by measuring Centrifugal Adhesion Balance
Table 2: Impact of adhesive and cohesive forces on contact angle and wettability |
|||
|---|---|---|---|
| Adhesive forces | Cohesive forces | Contact angle | Outcome |
| Strong adhesion | Weak cohesion | Low | Higher wettability,
Hydrophilic conditions |
| Weak adhesion | Strong cohesion | High | Poor wettability,
Hydrophobic conditions |
Data Source: Dr. Amita Joshi of Biology Online
-
Electrostatic forces of Adhesion
Conducting materials create a capacitor kind of structure at the surfaces due to the generation of differences in charge. The difference in charge at the surfaces results in the generation of electrostatic forces of adhesion.
-
Diffusive Forces of Adhesion
These forces of adhesion are seen when the two surfaces involved are mobile and have miscibility or solubility in each other. Such surfaces merge or bind together by diffusive forces of adhesion. It is usually seen during sintering wherein ceramic or metal surfaces are pressed and heated together, it results in the movement or diffusion of molecules from one surface to another resulting in the joining of the two surfaces. Diffusive forces of adhesion also come into play when one end of the polymer chain merges or diffuses into the other polymer. Diffusive forces of adhesion are similar to mechanical tethering, however, occurring at the molecular level.
Take the Quiz!
References
- Raos, G., & Zappone, B. (2021). Polymer Adhesion: Seeking New Solutions for an Old Problem#. Macromolecules, 54(23), 10617-10644.
- Coccolini, F., Ansaloni, L., Manfredi, R., Campanati, L., Poiasina, E., Bertoli, P., … & Catena, F. (2013). Peritoneal adhesion index (PAI): proposal of a score for the “ignored iceberg” of medicine and surgery. World Journal of Emergency Surgery, 8(1), 1-5.
- Marshall, S. J., Bayne, S. C., Baier, R., Tomsia, A. P., & Marshall, G. W. (2010). A review of adhesion science. Dental materials, 26(2), e11-e16.
- Lee, L. H. (Ed.). (2013). Fundamentals of adhesion. Springer Science & Business Media.
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.


