870608 | 4-HNE-dimethylacetal
(E)-4R-hydroxynonenal-dimethylacetal (Each vial contains 6.8mg which yields ~5.2mg 4-HNE after deprotection)
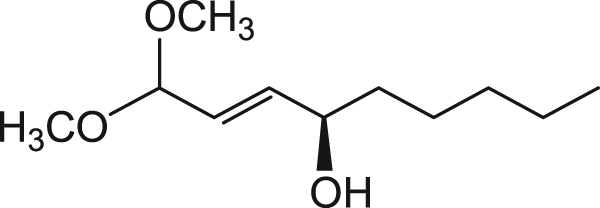
4-HNE-dimethylacetal
(E)-4R-hydroxynonenal-dimethylacetal (Each vial contains 6.8mg which yields ~5.2mg 4-HNE after deprotection)
CAS Registry Number is a Registered Trademark of the American Chemical Society
Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008 Sep 12;321(5895):1493-5. doi: 10.1126/science.1158554. PMID: 18787169; PMCID: PMC2741612.
PubMed ID: 18787169Awasthi YC, Yang Y, Tiwari NK, Patrick B, Sharma A, Li J, Awasthi S. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic Biol Med. 2004 Sep 1;37(5):607-19. doi: 10.1016/j.freeradbiomed.2004.05.033. PMID: 15288119.
PubMed ID: 15288119Sharma R, Brown D, Awasthi S, Yang Y, Sharma A, Patrick B, Saini MK, Singh SP, Zimniak P, Singh SV, Awasthi YC. Transfection with 4-hydroxynonenal-metabolizing glutathione S-transferase isozymes leads to phenotypic transformation and immortalization of adherent cells. Eur J Biochem. 2004 May;271(9):1690-701. doi: 10.1111/j.1432-1033.2004.04067.x. PMID: 15096208.
PubMed ID: 15096208Zarkovic N. 4-hydroxynonenal as a bioactive marker of pathophysiological processes. Mol Aspects Med. 2003 Aug-Oct;24(4-5):281-91. doi: 10.1016/s0098-2997(03)00023-2. PMID: 12893006.
PubMed ID: 12893006Zarkovic K. 4-hydroxynonenal and neurodegenerative diseases. Mol Aspects Med. 2003 Aug-Oct;24(4-5):293-303. doi: 10.1016/s0098-2997(03)00024-4. PMID: 12893007.
PubMed ID: 12893007Zarković N, Zarković K, Schaur RJ, Stolc S, Schlag G, Redl H, Waeg G, Borović S, Loncarić I, Jurić G, Hlavka V. 4-Hydroxynonenal as a second messenger of free radicals and growth modifying factor. Life Sci. 1999;65(18-19):1901-4. doi: 10.1016/s0024-3205(99)00444-0. PMID: 10576434.
PubMed ID: 10576434Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81-128. doi: 10.1016/0891-5849(91)90192-6. PMID: 1937131.
PubMed ID: 1937131- Certificate of Analysis (Lot No. 870608H-5MG-A-012 and 6212HHA012)
- Certificate of Analysis (Lot No. 870608H-5MG-A-012 and 6212HHA012)
